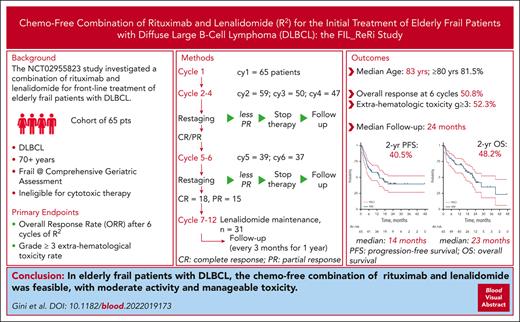
Key Points
Rituximab and lenalidomide combination is feasible and has moderate activity in frontline therapy of frail older patients with DLBCL.
The FIL_ReRi trial represents a benchmark for future studies devised for frail patients with DLBCL.
Abstract
Treatment of diffuse large B-cell lymphoma (DLBCL) in older patients is challenging, especially for those who are not eligible for anthracycline-containing regimens. Fondazione Italiana Linfomi (FIL) started the FIL_ReRi study, a 2-stage single-arm trial to investigate the activity and safety of the chemo-free combination of rituximab and lenalidomide (R2) in ≥70-year-old untreated frail patients with DLBCL. Frailty was prospectively defined using a simplified geriatric assessment tool. Patients were administered a maximum of 6 28-day cycles of 20 mg oral lenalidomide from days 2 to 22 and IV rituximab 375 mg/m2 on day 1, with response assessment after cycles 4 and 6. Patients with partial response or complete response (CR) at cycle 6 were administered lenalidomide 10 mg/d from days 1 to 21 for every 28 cycles for a total of 12 cycles or until progression or unacceptable toxicity. The primary end point was the overall response rate (ORR) after cycle 6; the coprimary end point was the rate of grade 3 or 4 extrahematological toxicity. The ORR was 50.8%, with 27.7% CR. After a median follow-up of 24 months, the median progression-free survival was 14 months, and the 2-year duration of response was 64%. Thirty-four patients experienced extrahematological toxicity according to the National Cancer Institute Common Terminology Criteria for Adverse Events grade ≥3. The activity of the R2 combination was observed in a significant proportion of subjects, warranting further exploration of a chemo-free approach in frail older patients with DLBCL. This trial was registered at EudraCT as #2015-003371-29 and clinicaltrials.gov as #NCT02955823.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most frequent B-cell non-Hodgkin lymphoma, accounting for 35%-40% of all lymphomas,1,2 with nearly one-third of the patients older than 75 years.3,4 DLBCL is associated with an aggressive natural history, and the outcomes have significantly improved since the monoclonal antibody anti-CD20 rituximab (R) was associated with anthracycline-containing regimens.5,6 Compared with those of their younger counterparts, the outcomes of older patients remain dismal because of age-related conditions and their reduced tolerance to treatment side effects.7,8 These issues have limited or excluded the older population from practice-changing clinical trials,9 preventing them from receiving new therapeutic approaches10 and increasing the risk of inadequate treatment, leaving the treating physician’s subjective assessment of the choice of therapy. In 2 historical series of patients older than 80 and 90 years, a dismal median overall survival (OS) of 1.3 years and 5.2 months, respectively, was observed, confirming more active therapies are needed in this population with a high unmet clinical need.11,12
In the last decade, geriatric assessment (GA) has been included in the initial assessment of older patients with cancer to capture age-specific vulnerabilities beyond those identified via routine assessments. This multidimensional approach evaluates physical and cognitive functions; comorbid conditions; socioeconomic, nutritional, and psychological status; the presence of geriatric syndromes; and the administration of repeated treatments. The purpose of GA is to provide an accurate characterization of the fitness status of older patients to tailor treatment intensity to their features.11-13 An analysis of 99 frail patients from a prospective trial treated with palliative R-chemotherapy reported a 5-year OS rate of 28%, which was much poorer than that of fit patients.14 In another prospective study of 173 patients aged >70 years, the 2-year OS improved via curative compared with palliative therapies only for fit or unfit patients, but not in the frail group.15 Recently, Merli et al16 described the outcomes of 1163 patients prospectively enrolled in the Fondazione Italiana Linfomi (FIL) FIL_Elderly project and evaluated them using simplified GA. Frail patients were associated with significantly low 3-year OS (43% vs 75% and 58% for frail vs fit and unfit, respectively) and were less likely to be treated with curative options (53% vs 99% and 84% for frail vs fit and unfit, respectively).
Lenalidomide monotherapy is associated with antilymphoma activity, has a manageable safety profile in relapsed refractory DLBCL,17-19 and has already achieved favorable results in DLBCL as well as in other lymphoma subtypes when combined with immunotherapy or chemotherapy agents.20-26
FIL_ReRi studied the activity and safety of a lenalidomide-rituximab combination (R2) in a population of older patients with a frail profile at the GA.
Patients and methods
Eligibility
Eligible patients were previously untreated, aged ≥70 years, and had histology-confirmed DLBCL,2 a frail profile at GA,15 an Eastern Cooperative Oncology Group performance status ≤3, and at least 1 bidimensionally measurable lesion ≥1.5 cm in its larger diameter on computed tomography (CT). Laboratory values should confirm adequate medullar and organ function (if not lymphoma-related). Patients were to have HIV-negative results; patients with hepatitis C virus infection were admitted, and patients with hepatitis B surface antigen–negative results and anti–hepatitis B core antibodies were enrolled if hepatitis B virus-DNA was undetectable and adequate antiviral treatment was given. Subjects with severe cardiac dysfunction (New York Heart Association grades 3-4), evidence of any severe active acute or chronic infection, and absence of a caregiver in nonautonomous patients constituted the main exclusion criteria. Cell of origin (COO) was retrospectively defined via immunohistochemical staining, using the Hans algorithm method.27
Study design and treatment
FIL_ReRi was a prospective multicenter, 2-stage, single-arm, phase 2 trial. Patients received 4 or 6 28-day cycles of R2 according to the following: cycle 1, IV rituximab 375 mg/m2 on days 1, 8, and 15, oral dexamethasone 5 mg on days 1, 8, 15, and 22 to reduce tumor burden, and oral lenalidomide 15 mg once a day from days 2 to 22. In cycles from 2 to 4, IV rituximab 375 mg/m2 on day 1 and oral lenalidomide 20 mg once a day from days 2 to 22 were administered (supplemental Table 1, available on the Blood website). Lenalidomide dose was modulated based on hematological toxicity, whereas the rituximab dose was not, with treatment delayed in case of need (supplemental Table 2). Patients achieving a partial (PR) or complete (CR) response after 4 cycles received 2 additional cycles. Patients with ≤PR after 4 or 6 cycles discontinued the study treatment and were managed at the physician’s discretion. Patients with ≥PR after 6 cycles received maintenance oral lenalidomide 10 mg/d from days 1 to 21 of 28-day cycles, until a maximum of 12 cycles (including induction), progression, or unacceptable toxicity, whichever came first. The delivered dose intensity was calculated according to Hryniuk.28 All patients, including those with early withdrawals, were followed up every 3 months for 1 year. An extended 5-year follow-up after the end of the study was planned, which required sites to only provide information on patient status and possible events occurring after the end of the study, including the diagnosis of second neoplasia and long-term toxicity.
FIL_ReRi was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice Standards at 18 FIL centers after the approval of the relevant ethics committees. Written informed consent was obtained from all patients.
Assessments
Patients were screened over a 30-day period with complete physical examination, vital signs and Eastern Cooperative Oncology Group performance status measurements, hematological and blood chemistry tests, disease evaluation via CT, and frailty status according to GA. The first clinical/radiological assessment of the target lesions was performed after cycle 4 and again after cycle 6, with complete restaging. The primary end point was the overall response rate (ORR) via a CT, which was measured according to the 2007 revised criteria.29 The final response was evaluated within 28 days of the last study drug administration. [18F]fluorodeoxyglucose-positron emission tomography whole-body scan and bone marrow biopsy were recommended but not mandatory. Safety was assessed by recording the frequency and severity of adverse events (AEs) classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. The principal measure of safety was clinically relevant toxicity, defined as the proportion of patients experiencing at least 1 extrahematological grade ≥3 AE. The worst toxicity per organ and per cycle was considered. The secondary end points included the CR rate, progression-free survival (PFS), OS, duration of response (DoR), dropout rate, and rate of treatment discontinuation because of toxicity or treatment intolerance.29
Statistical analysis
An optimal Simon 2-stage design30 with 61 evaluable patients provided 90% power to test the null hypothesis that the ORR is at most 45% vs the alternative hypothesis that the ORR is at least 65%, with a significance level of α = 0.05 (2-sided). As for the interim analysis at the end of stage 1, the study was to be closed to enrollments until the required 23 patients were evaluable for response assessment: at least 12 responses (PR/CR) were to be recorded to proceed with the accrual of additional 38 evaluable patients in stage 2 for a total of 61 evaluable patients. In the final analysis, at least 34 responses were required to consider the primary end point met. Seven additional patients were enrolled to account for dropouts from a total of 68 patients. Extrahematological toxicity grade ≥3 was monitored according to the Ray and Rai methods.31 The boundary value was defined as the alpha-spending function corresponding to the O’Brien-Fleming boundary values. With a maximum tolerated extrahematological toxicity grade ≥3 rate of 35%, an α error of 0.05 and, according to the previously defined sample size, a maximum of 14 toxic events in the first 23 patients enrolled were tolerated to continue the study, and a maximum of 27 in the 61 evaluable patients was required to consider the study safe. All patients who received at least 1 dose of R2 were considered evaluable for efficacy and safety and monitored since the time of their first treatment administration. The best response achieved by each patient during induction was considered. ORR was calculated as the percentage of patients with at least 1 PR; ORR point and relative 90% confidence interval (CI) were estimated according to the Koyama-Chen approach,32 which takes into account the 2-stage design of the study. Because of the 2-stage design, the 95% CI for ORR was adjusted according to Atkinson and Brown.33 Additional details are provided in the supplemental Data. The data were centralized by the sponsor. The statistical analysis was performed at the Unit of Statistics of the FIL operational offices, which also contributed to the design. Data and statistical analyses were fully available to all authors, who checked their accuracy, completion, integrity, and adherence to the protocol. All authors contributed to and reviewed the subsequent drafts and jointly decided to submit the manuscript for publication. The corresponding author had the final responsibility for submitting the manuscript for publication.
Results
Patients and treatment
From January 2017 to June 2021, 68 patients were enrolled (Figure 1). Three patients were subsequently excluded because of withdrawal of consent (1), presence of hepatic dysfunction (1), and death before treatment start (1). Sixty-five patients were confirmed eligible and started treatment. The patient characteristics are reported in Table 1. The median number of induction cycles administered was 6 (range, 1-6), as was the case for maintenance treatment. Twenty-two patients (33.8%) received 1 year of total treatment. The mean dose intensity of rituximab was 0.988, 0.998, and 0.999 on days 1, 8, and 15, respectively. The median dose intensities of lenalidomide were 0.943, 0.750, and 1.00, during cycle 1, from cycles 2 to 6, and maintenance, respectively. During the first cycle, 21% and 74% of patients received from 50% to 75% and >75% of the planned lenalidomide dose, respectively; between cycles 2 and 6, a lenalidomide dose from 50% to 75%, and >75% was administered in 34% and 52% of the patients, respectively; during the maintenance phase, 93% of patients were treated with a dose >75%. Treatment delay during induction occurred in 27 of 65 patients who started induction (41.5%) and in 19 of 31 patients who received maintenance (61.2%). The median delay was 7 additional days with respect to the planned treatment schedule.
Study profile. Flowchart of the patients included in the study from screening to EOI. CR, complete response; PD, progressive disease; PET, positron emission tomography; PR, partial response; SD, stable disease; TX, treatment.
Study profile. Flowchart of the patients included in the study from screening to EOI. CR, complete response; PD, progressive disease; PET, positron emission tomography; PR, partial response; SD, stable disease; TX, treatment.
Baseline patient characteristics (N = 65)
| Characteristic . | No. . | Percentage . |
|---|---|---|
| Median age, y (range) | 83 (70-91) | |
| Age, y | ||
| 70-79 | 12 | 18.5 |
| 80-84 | 30 | 46.1 |
| >85 | 23 | 35.4 |
| Median hemoglobin level, g/dL (range) | 12.3 (7.6-15.3) | |
| Median creatinine level, mg/dL (range) | 0.9 (0.4-3.5) | |
| Median WBC level, 109/L (range) | 7.0 (1.3-28.5) | |
| Median ANC, 109/L (range) | 5.0 (0.2-24.0) | |
| Sex, male | 30 | 46.1 |
| Systemic symptoms presence | 21 | 33.3 |
| ECOG PS >1 | 15 | 23.1 |
| Stage III-IV | 47 | 72.3 |
| ENS >1 | 14 | 21.5 |
| Largest diameter of lymph nodes >6 cm | 20 | 32.8 |
| LDH > ULN | 32 | 51.6 |
| IPI 3-5 | 35 | 56.4 |
| EPI | ||
| Intermediate risk | 22 | 36 |
| High risk | 39 | 64 |
| HBV serology status∗ | ||
| HBsAb neg/HbcAb neg | 37 | 65.0 |
| HBsAb neg/HBcAb pos (1 HBV-DNA pos) | 6 | 10.5 |
| HBsAb pos/HBcAb neg | 3 | 5.2 |
| HBsAb pos/HBcAb pos | 11 | 19.3 |
| HBV serology positive (total) | 20 | 35 |
| ADL score | ||
| 6 | 36 | 59 |
| 5 | 51 | 34 |
| ≤4 | 4 | 7 |
| IADL score | ||
| 8 | 15 | 25 |
| 6-7 | 38 | 62 |
| ≤5 | 8 | 13 |
| CIRS | ||
| Number of comorbidities grade ≥2: | ||
| 0/4 | 34 | 56 |
| 5 | 18 | 29 |
| 6/8 | 9 | 15 |
| Number of comorbidities grade ≥3: | ||
| 1 | 5 | 8 |
| 2 | 1 | 2 |
| COO based on Hans algorithm | ||
| GCB | 29 | 48 |
| Non-GCB | 32 | 52 |
| Characteristic . | No. . | Percentage . |
|---|---|---|
| Median age, y (range) | 83 (70-91) | |
| Age, y | ||
| 70-79 | 12 | 18.5 |
| 80-84 | 30 | 46.1 |
| >85 | 23 | 35.4 |
| Median hemoglobin level, g/dL (range) | 12.3 (7.6-15.3) | |
| Median creatinine level, mg/dL (range) | 0.9 (0.4-3.5) | |
| Median WBC level, 109/L (range) | 7.0 (1.3-28.5) | |
| Median ANC, 109/L (range) | 5.0 (0.2-24.0) | |
| Sex, male | 30 | 46.1 |
| Systemic symptoms presence | 21 | 33.3 |
| ECOG PS >1 | 15 | 23.1 |
| Stage III-IV | 47 | 72.3 |
| ENS >1 | 14 | 21.5 |
| Largest diameter of lymph nodes >6 cm | 20 | 32.8 |
| LDH > ULN | 32 | 51.6 |
| IPI 3-5 | 35 | 56.4 |
| EPI | ||
| Intermediate risk | 22 | 36 |
| High risk | 39 | 64 |
| HBV serology status∗ | ||
| HBsAb neg/HbcAb neg | 37 | 65.0 |
| HBsAb neg/HBcAb pos (1 HBV-DNA pos) | 6 | 10.5 |
| HBsAb pos/HBcAb neg | 3 | 5.2 |
| HBsAb pos/HBcAb pos | 11 | 19.3 |
| HBV serology positive (total) | 20 | 35 |
| ADL score | ||
| 6 | 36 | 59 |
| 5 | 51 | 34 |
| ≤4 | 4 | 7 |
| IADL score | ||
| 8 | 15 | 25 |
| 6-7 | 38 | 62 |
| ≤5 | 8 | 13 |
| CIRS | ||
| Number of comorbidities grade ≥2: | ||
| 0/4 | 34 | 56 |
| 5 | 18 | 29 |
| 6/8 | 9 | 15 |
| Number of comorbidities grade ≥3: | ||
| 1 | 5 | 8 |
| 2 | 1 | 2 |
| COO based on Hans algorithm | ||
| GCB | 29 | 48 |
| Non-GCB | 32 | 52 |
Percentages may not correspond to 100% because of rounding.
ADL, 6 items; IADL, 8 items.
ADL, activities of daily living; ANC, absolute neutrophil count; CIRS, Cumulative Illness Rating Scale; ECOG PS, Eastern Cooperative Oncology Group performance status; ENS, extranodal site of involvement; EPI, Elderly Project Index; HBc, hepatitis B core (antigen); HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; IADL, instrumental activities of daily living; IPI, international prognostic index; neg, negative; LDH, lactate dehydrogenase; pos, positive; ULN, upper limit of normal; WBC, white blood cell.
All 65 patients tested HBsAg negative; serology was tested in 57 patients.
Interim analysis
The accrual of the first 23 patients needed for study stage 1 was completed in December 2017, and a programmed interim analysis was performed. Twelve patients achieved a response on CT, with an ORR greater than the lower limit for the futility of 11 responses. Thirteen extrahematological National Cancer Institute Common Terminology Criteria for Adverse Events grade ≥3 AEs were recorded, which was below the upper limit for the futility of 15 relevant toxicity events. The study was then reopened to enrollment in July 2018 until the registration of the planned 68 patients.
Efficacy
Induction treatment was completed in 37 (56.9%) patients. At restaging at the end of induction (EOI), 33 patients achieved a response: 18 (27.7%) patients were reported to be in CR and 15 in PR (1 patient reached PR after 5 cycles) for an ORR of 50.8% (95% CI, 38.1-63.4). The ORR point estimate according to the Koyama-Chen approach was 58.4% (90% CI, 43.7-67.1; P = .075, with null hypothesis = 45%). A dropout rate of 43.1% was reported during induction, with treatment prematurely discontinued in 28 patients because of lymphoma progression (7), extrahematological toxicity (5), hematological toxicity (2), patient discontinued treatment after the first cycle (1), or lost due to death (13: infection, 1; progression, 2; infection/progression, 1; pancytopenia/heart failure, 1; cachexia/progressive disease, 2; arterial ischemia, 3; kidney cancer, 1; dehydration, 1; and unknown cause, 1). The rate of treatment discontinuation because of toxicity only was 21.5%, corresponding to half of all dropouts.
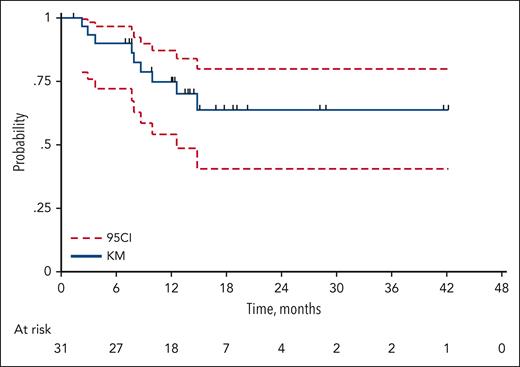
After induction, 31 patients received lenalidomide maintenance for a maximum duration of treatment, including the induction phase of 12 cycles (range, 8-12); 2 patients in PR/CR at EOI were not administered with maintenance, 1 because of physician decision and the other, in PR, because of study interruption after cycle 5. At restaging after maintenance, 11 patients (35.5%) were reported to be in CR and 6 (19.3%) in PR. After maintenance, the conditions of 2 patients converted from CR to PR, whereas all other patients maintained the response of EOI.
After a median follow-up of 24 months (range, 1-47), 36 events for PFS were recorded (23 progressive disease and 13 deaths from any cause), with a 2-year PFS of 40.5% (95% CI, 27.6-53.0) and a median duration of PFS of 14.0 months (95% CI, from 6.8 to not reached; Figure 2). Median DoR was not reached; 2-year DoR was 64.6% (95% CI, 42.1-80.1; Figure 3). Overall, 32 deaths were recorded, resulting in a 2-year OS of 48.2% (95% CI, 33.4-61.1) and a median of 23.2 months (95% CI, 16.3-38.9; Figure 4). The main cause of death was lymphoma progression, which was reported in 14 subjects (43.8% of deaths); other causes are reported in supplemental Table 3. At the time the ReRi study was opened, the Elderly Project Index34 had not yet been defined; nevertheless, 61 patients had the required parameters to calculate the index. No difference was observed in the OS and PFS between patients with intermediate- and high-risk EPI groups (supplemental Table 4). Sixty-one patients were available for COO analysis: 48% and 52% were classified as having germinal center B-cell DLBCL (GCB) and non-GCB, respectively. ORR by COO was 16 of 32 (50%) in the non-GCB group and 14 of 29 (48%) in the GCB group (P = .900), with 11 (34%) and 6 (21%) CRs among non-GCB and GCB groups, respectively (P = .266). Neither PFS nor OS were affected by COO (log-rank test, P = .660 and P = .632, respectively).
PFS. Total of 36 events (progressions n = 23; deaths from any cause n = 23); 2-year PFS 39.6% (95% CI, 26.6-52.4), median PFS 14.0 months (95% CI, from 6.8 to not reached). The blue solid line represents the PFS probability according to the Kaplan-Meier (KM) method and the red dashed line the 95% confidence interval (95CI).
PFS. Total of 36 events (progressions n = 23; deaths from any cause n = 23); 2-year PFS 39.6% (95% CI, 26.6-52.4), median PFS 14.0 months (95% CI, from 6.8 to not reached). The blue solid line represents the PFS probability according to the Kaplan-Meier (KM) method and the red dashed line the 95% confidence interval (95CI).
Duration of response (DoR). Thirty-one of the 33 patients eligible for maintenance started maintenance after induction. A total of 9 events (progressions, n = 8; deaths from any cause, n = 1); 2-year DoR, 63.8% (95% CI, 40.6-79.9), median not reached. The blue solid line represents the DoR probability according to the Kaplan-Meier (KM) method and the red dashed line the 95% confidence interval (95CI).
Duration of response (DoR). Thirty-one of the 33 patients eligible for maintenance started maintenance after induction. A total of 9 events (progressions, n = 8; deaths from any cause, n = 1); 2-year DoR, 63.8% (95% CI, 40.6-79.9), median not reached. The blue solid line represents the DoR probability according to the Kaplan-Meier (KM) method and the red dashed line the 95% confidence interval (95CI).
OS. Median follow-up of 21.8 months (95% CI, 18.4-24.8 months), ranging from 1 to 47 months. Total of 32 events (lymphoma, n = 18 [56.2%]); 2-year OS 47.5% (95% CI, 30.7-59.6), median OS 22.0 months (95% CI, 16.4-38.9). The blue solid line represents the OS probability according to the Kaplan-Meier (KM) method and the red dashed line the 95% confidence interval (95CI).
OS. Median follow-up of 21.8 months (95% CI, 18.4-24.8 months), ranging from 1 to 47 months. Total of 32 events (lymphoma, n = 18 [56.2%]); 2-year OS 47.5% (95% CI, 30.7-59.6), median OS 22.0 months (95% CI, 16.4-38.9). The blue solid line represents the OS probability according to the Kaplan-Meier (KM) method and the red dashed line the 95% confidence interval (95CI).
Safety
Considering the whole population, grade ≥3 neutropenia and grade ≥3 infections were reported in 46% and 10% of the patients, respectively. During induction, at least 1 dose of prophylactic granulocyte-colony stimulating factors was administered to 30 patients (46%). Table 2 lists the recorded toxicity events: overall, 34 patients out of the 65 analyzed (52.3%; 95% CI, 40.0-64.9) experienced at least 1 grade ≥3 extrahematological toxicity event. The most relevant toxicities were respiratory, general, and skin/subcutaneous disorders (7 events each), cardiac and vascular disorders (6 events each), and infections (5). Overall, 37 serious adverse events and 13 suspected unexpected serious adverse reactions were recorded in the 36 eligible patients.
Hematological and extrahematological grade greater than or equal to 3 AEs
| . | Grade 3 . | Grade 4 . | Grade 5 . | |||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | |
| Any hematological toxicity | 27 | 11 | — | |||
| Anemia | 2 | 3.1 | — | — | ||
| Neutropenia | 21 | 32.3 | 9 | 13.8 | — | |
| Thrombocytopenia | 4 | 6.2 | 2 | 3.1 | — | |
| Any extrahematological toxicity | 41 | 4 | 5 | |||
| Respiratory/thoracic and mediastinal disorders | 5 | 10.0 | 1 | 2.0 | 1 | 2.0 |
| General disorders/administration site conditions | 6 | 12.0 | 1 | 2.0 | — | |
| Skin and subcutaneous tissue disorders | 6 | 12.0 | 1 | 2.0 | — | |
| Cardiac disorders | 5 | 10.0 | — | 1 | 2.0 | |
| Vascular disorders | 3 | 6.0 | — | 3 | 6.0 | |
| Infections | 3 | 6.0 | 1 | 2.0 | — | |
| Gastrointestinal disorders | 3 | 6.0 | — | — | ||
| Nervous system disorders | 3 | 6.0 | — | — | ||
| Investigations | 1 | 2.0 | — | — | ||
| Metabolism and nutrition disorders | 2 | 4.0 | — | — | ||
| Musculoskeletal and connective tissue disorders | 2 | 4.0 | — | — | ||
| Neoplasms benign/malignant/unspecified | 1 | 2.0 | — | — | ||
| Psychiatric disorders | 1 | 2.0 | — | — | ||
| . | Grade 3 . | Grade 4 . | Grade 5 . | |||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | |
| Any hematological toxicity | 27 | 11 | — | |||
| Anemia | 2 | 3.1 | — | — | ||
| Neutropenia | 21 | 32.3 | 9 | 13.8 | — | |
| Thrombocytopenia | 4 | 6.2 | 2 | 3.1 | — | |
| Any extrahematological toxicity | 41 | 4 | 5 | |||
| Respiratory/thoracic and mediastinal disorders | 5 | 10.0 | 1 | 2.0 | 1 | 2.0 |
| General disorders/administration site conditions | 6 | 12.0 | 1 | 2.0 | — | |
| Skin and subcutaneous tissue disorders | 6 | 12.0 | 1 | 2.0 | — | |
| Cardiac disorders | 5 | 10.0 | — | 1 | 2.0 | |
| Vascular disorders | 3 | 6.0 | — | 3 | 6.0 | |
| Infections | 3 | 6.0 | 1 | 2.0 | — | |
| Gastrointestinal disorders | 3 | 6.0 | — | — | ||
| Nervous system disorders | 3 | 6.0 | — | — | ||
| Investigations | 1 | 2.0 | — | — | ||
| Metabolism and nutrition disorders | 2 | 4.0 | — | — | ||
| Musculoskeletal and connective tissue disorders | 2 | 4.0 | — | — | ||
| Neoplasms benign/malignant/unspecified | 1 | 2.0 | — | — | ||
| Psychiatric disorders | 1 | 2.0 | — | — | ||
Hematological toxicity values and percentages refer to the 38 events recorded in the total population of 65 patients. Extrahematological toxicity values and percentages refer to the 50 events recorded in 34 patients (1 patient may have experienced more than 1 grade ≥3 AE). The percentages may not correspond to 100% because of rounding.
Discussion
In this study, we investigated the activity and safety or the R2 combination in a population of older patients with DLBCL prospectively defined as frail according to GA. R2 was feasible, and despite the fact that primary goals were not met, the activity of the R2 was valuable, with the ORR and complete response rate being 50.7% and 23.1%, respectively. Moreover, the median PFS was 14 months, the median OS was 22 months, and a favorable 2-year DoR of 63.8% in patients who achieved at least PR was estimated. These results are encouraging because 82% of the patients enrolled were >80 years old, and none were eligible for conventional aggressive treatment because of frailty.
A key and innovative aspect of our study relies on the prospective definition of ineligibility for anthracycline-containing regimens, which was based on the prospective use of the validated GA tool. This approach was based on previous findings from our group that confirmed dismal outcomes for older patients with DLBCL who were classified as frail using the GA tool, even if they were treated with anthracyclines at reduced doses, and helped indentify an unmet need in the search of active therapies in this subset of patients.14-16 To confirm that the use of GA was effective in identifying patients who were not eligible for curative options, most patients whose condition progressed after ReRi in our study did not receive a second-line therapy. Among the 7 patients who relapsed after R2, 2 died before any salvage therapy could be administered, and only 1 of the 5 remaining patients was treated with an anthracycline-containing regimen.
Results of studies from other groups can hardly be compared with ours because they all addressed patients eligible for anthracycline-containing regimens. In 2 prospective trials with an anti-CD20 monoclonal antibody, rituximab or ofatumumab, combined with reduced-dose cyclophosphamide, doxorubicin, vincristine, and prednisone (mini-CHOP), all patients had to receive anthracyclines even if at reduced doses.35,36 Both studies showed good results in terms of efficacy but also showed increased mortality due to non-lymphoma–related events, which were likely mitigated by the use of a prephase treatment in the second trial with ofatumumab-mini-CHOP.36 Moreover, limitations in instrumental activities of daily living, which were reported in 53% of the patients treated with R-mini-CHOP, identified subjects with reduced survival (median OS, 26 months), suggesting that the use of functional scales has an important prognostic role in the management of older patients with DLBCL.35
Using the same selection criteria as those adopted in the ReRi study, a previous phase 2 study of Fondazione Italiana Linfomi (FIL_R-BENDA FRAIL) investigated the combination of rituximab and bendamustine (R-B) as an initial therapy in frail older patients (Storti et al37). This study dealt with a population whose characteristics were superimposable on that of FIL_ReRi. Forty-five patients were included and treated with 4 or 6 cycles of R-B, depending on the age-adjusted International Prognostic Index, followed by 2 additional cycles of R. The observed ORR was 62%, with a 53% CR; the 2-year PFS was 38%, with a median of 10 months; and the 2-year OS was 51%, with a median of 30 months. Even if R-B showed higher response rates than R2, the activity and efficacy of the 2 regimens were similar, although R2 did not include cytotoxic agents.
With respect to safety, a considerable proportion of patients required dose reduction of lenalidomide or a delay in treatment administration, which correlated with a higher-than-expected proportion of toxic events. However, it must be stressed that even if the protocol included recommendations for lenalidomide dose reduction, the use of prophylactic measures was left to the treating physicians. An incorrect or insufficient use of preventive therapies could, in part, explain the high rate of AEs recorded.
Additional efforts are needed to outline a treatment leading to increased response rates, improved toxicity profiles, and prolonged DoR among frail patients. The chemo-free approach could likely be the way forward for better treatment among frail older patients with DLBCL. The use of chemo-free schedules is now under investigation in a variety of tumors, including lymphomas, given the recent availability of novel agents, including bispecific antibodies, antibody-drug conjugates, and cellular therapies. Among the new options that may be translated into future therapies for frail patients, Westin et al38 reported the final results of the Smart Start trial. The study was devised for patients with non-GCB DLBCL who were initially treated with a short 2-month induction therapy with rituximab, lenalidomide, and ibrutinib, followed by standard immunochemotherapy. The 52 patients considered evaluable after enrollment had a median age of 64 years and were all fit for chemotherapy; nevertheless, the results of the initial 2 cycles of chemo-free treatment were remarkable, with an ORR of 84.6% and a complete response rate of 38.5%. One patient refused to proceed with preplanned chemotherapy after achieving a CR with 2 cycles of rituximab, lenalidomide, and ibrutinib and was relapse-free, with no additional therapy 18 months later. Despite the fact that this population was not comparable with ours, this study showed the high activity of a novel chemo-free combination for patients with DLBCL and might be used as a basis to develop a treatment suitable for the frail patient subset. Studies are also ongoing using mosunetuzumab (mosun), a full-length, humanized, immunoglobulin G1 CD20 × CD3 bispecific antibody. Favorable efficacy and tolerable safety profiles have been shown with mosun administered as a single agent for relapsed/refractory B-cell lymphomas in a phase 1 study39 (NCT02500407). Moreover, updated data from the phase 1/2, multicenter GO40554 study (NCT03677154)40 confirmed the notable efficacy and tolerability of first-line mosun monotherapy in older/unfit patients with DLBCL; eligible subjects were aged ≥80 years or from 60 to 79 years, with impairment in ≥1 activity of daily living or instrumental activity of daily living or reduced cardiac, renal, or liver function, precluding the use of full-dose immunochemotherapy. No fatal AEs were observed. Common treatment-emergent AEs (>10%) included cytokine release syndrome (n = 9; 22.5%), abdominal pain (n = 7; 17.5%), rash (n = 5; 12.5%), and neutropenia (n = 5; 12.5%). The ORR in efficacy-evaluable patients (n = 31) was 67.7%, and the CR rate was 41.9%, which favorably compare with the results of our study. Zhang et al41 evaluated the safety and efficacy of chimeric antigen receptor T-cell therapy in older patients with relapsed/refractory DLBCL based on the Comprehensive Geriatric Assessment system. In this study, 31 patients older than 65 years were enrolled and classified as fit (17), unfit (10), and frail (4) according to the Comprehensive Geriatric Assessment. The unfit/frail group achieved ORR, CR, and PR rates of 64.3%, 42.9%, and 21.4%, respectively; the median PFS and OS were 7 and 11 months, respectively, suggesting the feasibility of chimeric antigen receptor T-cell therapy in this hard-to-treat population.
At least equally important to treatment is the identification of patients who are not eligible to receive conventional curative options. The Storti et al study,37 along with the results of this study and the validation study by Merli et al16 have been able to identify a population of frail patients who had never been represented in clinical trials before and to set activity and safety bars to define the role of immunochemotherapy and chemo-free options in this population. In this context, the aforementioned trials confirm the feasibility and reproducibility of the FIL GA tool to define the frailty status and suggest the use of this tool for future research addressing the unmet medical needs of frail patients.
Some limitations of our study should be considered. The first issue is represented by the initial unplanned collection of biological data and, in particular, details on the COO. Indeed, COO profiling was added after the study opened as a secondary study end point, and the analysis of results by COO profile was ongoing at the time of writing this manuscript. However, because few samples were available, we consider it unlikely to achieve firm conclusions based on the COO type. Secondly, we should consider that the study result was formally negative, and the minimum number of responses required by the statistical plan to confirm that the alternative hypothesis was not reached. However, when the ReRi trial was designed, assumptions were based on the results achieved with lenalidomide with/without rituximab in the relapsed setting of patients with DLBCL, showing high activity even in older and heavily pretreated patients, which led us to make an unduly optimistic hypothesis. Using a different and more realistic hypothesis, the ReRi results might have been read as positive. Finally, in our study, we did not plan strict follow-up monitoring using CTs because of the frailty status of the patients. This decision might have reduced the accuracy of the PFS assessment, which was identified as a secondary end point, with a limited impact on the overall study results.
Having acknowledged the study limitations, we still believe that our results are worth presenting because they show an activity, moderate yet of a chemo-free combination in a population of patients who lack curative options. In the absence of a randomized comparison, we could not draw any conclusions on the relative efficacy of the R2 regimen with other immunochemotherapy options, and we are far from issuing any recommendations. We are also aware of the fact that GA does not fully account for eligibility for anthracycline-containing regimens, but we trust that our approach to patient selection is the first attempt to objectively define criteria to tailor treatment intensity in a challenging and otherwise heterogeneous patient population. Moreover, we believe that the ReRi trial, together with the previous FIL trial with R-bendamustine among a frail population defined using the same criteria adopted for the ReRi, generated data that allowed the setting of a reference for treatment activity in a patient population so far otherwise hardly investigated with more conventional approaches.
In conclusion, FIL_ReRi is among the first studies to evaluate the activity and safety of chemo-free therapy in the frontline setting of patients with DLBCL who are not eligible for conventional cytotoxic therapy. The activity of the R2 combination was observed in a significant proportion of cases, warranting further exploration of chemo-free approaches in this setting, and our results could constitute a new benchmark for future studies.
Acknowledgments
The authors are grateful to the sponsor Fondazione Italiana Linfomi and to the personnel of its operational offices, particularly Maria Antonella Ferranti, and thank Daniela Gioia and Alessandro Levis for the study pharmacovigilance. The authors acknowledge Monica Bellei (a salaried employee of Fondazione Italiana Linfomi) for medical writing during the preparation of the manuscript. The authors are indebted to Gaia Goteri for histological revision of the patient specimens and COO profiling. Lenalidomide was unconditionally provided by Celgene.
None of the funders of the study had a role in the study design, data analysis, interpretation, or manuscript writing or submission. The authors directed the development of the manuscript and are fully responsible for all the content and editorial decisions regarding this manuscript.
Authorship
Contribution: G.G., M.T., L.M., F.M., V.R.Z., G.M., A.F., and S.L. conceived and designed the research; G.G., L.M., M. Cesaretti, M.B., and S.L. analyzed and interpreted the data; G.G., S.L., and M.B. wrote the manuscript; G.G., M.T., A.T., A.P., F.B., M.P., F.M., A.O., F.L., O.A., V.R.Z., A.M.L., M.C.T., A.A., D. Marino, G.M., S.P., D. Mannina, C.P., M. Celli., and S.L. enrolled patients in the study and collected data; and all authors reviewed the manuscript before submission.
Conflict-of-interest-disclosure: G.G. received consulting fees from Kyowa Kirin and Incyte, payment or honoraria for lectures/speakers bureaus/educational events from Kite, Takeda, Novartis, Gentili, Incyte, and Janssen; support for attending meeting and/or travel from AbbVie and Janssen; and participation in the data safety monitoring board/advisory board for Takeda, Gentili, and Kite. A.T. received payment or honoraria for lectures/speakers bureaus/educational events from Takeda, Kyowa Kirin, and MSD; support for attending meetings and/or travel from Takeda, and Janssen; and participation in the data safety monitoring board/advisory board for Janssen, Gentili, and Sanofi. L.M. provided expert consultancy to Sandoz (unpaid). F.M. received support for attending meeting and/or travel from Takeda and Janssen; participation in the data safety monitoring board/advisory board for Janssen, Gilead, Takeda, Novartis, and Roche; and leadership role in Fondazione Italiana Linfomi Onlus (nonprofit organization, unpaid) and GRADE Onlus (nonprofit organization, unpaid). O.A. received payment or honoraria for lectures/speakers bureaus/educational events from Amgen and Janssen Cilag; and support for attending meetings and/or travel from Janssen Cilag, Gilead, and Takeda. V.R.Z. received consulting fees from Roche. A.M.L. received support to the study reported in the present manuscript from Bristol Myers Squibb; grants/contracts from Takeda, Servier, Roche, Celgene, AbbVie, Incyte, Janssen, Sanofi, Verastem, Novartis, MorphoSys, GSK, Oncopeptides, Karyopharm, Onconova, Archigen, Pfizer, and FibroGen; consulting fees from Incyte; payment or honoraria for lectures/speakers bureaus/educational events from Iqvia, Servier, Celgene, AbbVie, Bristol Myers Squibb, and Janssen; support for attending meetings and/or travel from Takeda, Roche, Incyte, Janssen, Celgene, Bristol Myers Squibb, AbbVie, Novartis, Sanofi, Iqvia, and Verastem; and participation in the data safety monitoring board/advisory board for Amgen and Servier. M.C.T. received payment or honoraria for lectures/speakers bureaus/educational events from Gilead, Novartis, Bristol Myers Squibb, Janssen, and Incyte; and participation in the data safety monitoring board/advisory board for Incyte, Bristol Myers Squibb, Gilead, and Novartis. A.A. received payment or honoraria for lectures/speakers bureaus/educational events from Janssen, AbbVie, and Novartis; support for attending meetings and/or travel from Janssen, Gilead, and Takeda; and participation in the data safety monitoring board/advisory board for Janssen. G.M. received consulting fees from Janssen, Incyte, Roche, and AbbVie; payment or honoraria for lectures/speakers bureaus/educational events from Janssen, Incyte, Roche, and AbbVie; and support for attending meetings and/or travel from Janssen and AbbVie. A.F. received payment or honoraria for lectures/speakers bureaus/educational events from Servier and Kyowa Kirin; support for attending meetings and/or travel from Takeda; and participation in the data safety monitoring board/advisory board for Roche, Janssen, and Incyte. S.L. received consulting fees from GenMAb, Regeneron, Incyte, Takeda; payment or honoraria for lectures/speakers bureaus/educational events from Janssen and Bristol Myers Squibb; and participation in the data safety monitoring board/advisory board for Roche, Janssen, Bristol Myers Squibb, Gilead, Kite, Regeneron, Takeda, and GenMAb. The remaining authors declare no competing financial interests.
Correspondence: Guido Gini, Struttura Organizzativa Dipartimentale di Clinica Ematologica, Azienda Ospedaliero Universitaria delle Marche - Università Politecnica delle Marche, Ospedali Riuniti di Ancona, Via Conca 71, 60100 Ancona, Italy; e-mail: guido.gini@ospedaliriuniti.marche.it.
References
Author notes
Presented orally in abstract form at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 11 to 14 December 2021.
The study protocol and statistical analysis plan, for what is not already available in the supplemental Data, and individual-level patient clinical data reported in this manuscript (pseudonymized) are available on request from Fondazione Italiana Linfomi (segreteriadirezione@filinf.it). Requests will be reviewed based on scientific merit.
The online version of this article contains a data supplement.
A complete list of the sites of Fondazione Italiana Linfomi that took part in the study by enrolling patients appears in the supplemental Data.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



![OS. Median follow-up of 21.8 months (95% CI, 18.4-24.8 months), ranging from 1 to 47 months. Total of 32 events (lymphoma, n = 18 [56.2%]); 2-year OS 47.5% (95% CI, 30.7-59.6), median OS 22.0 months (95% CI, 16.4-38.9). The blue solid line represents the OS probability according to the Kaplan-Meier (KM) method and the red dashed line the 95% confidence interval (95CI).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/17/10.1182_blood.2022019173/2/m_blood_bld-2022-019173-gr4.jpeg?Expires=1764962316&Signature=4Kor1ULpGuN~x0Xn~ugq2gsaWVmlzcEUU81LvC83dU2u6yK5XfDRmqvvMKx7AKZIyBMitoefL~fcAQztJqDdP3rc38~bqrcVheKEbO-HJ2S5xP2ARoV~dKgP7hp76p9RaRSB6wBOMteJnwwJU5xWrbzsfN4oH2Oa2B-iB4qFcf5-ugPYlNxM2H68WXNUFfijzkR7exxWmhwQtbMAP5TJ6eEVrm6mjQlRMMf~kE-wIyed9XqFM~4AwXvdywzOVMef7GIu2~ygzrxzZdYVYAHyj4wm3aVVds00A8IZ1CA0mspDoDg7EVkRAc64x429ga40Va820z3YUaa6t~wV~Q2mkQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal