Abstract
Multiple myeloma remains an incurable disease plagued by high relapse rates. Deeper and more sustainable responses, however, have been consistently shown to improve outcomes and could eventually pave the way to achieving a cure. Our understanding of disease response has surpassed complete response (CR), because the current definitions are suboptimal, and the treatment goal should aim even beyond stringent CR, toward molecular and flow CR, that is, measurable residual disease (MRD) negativity. It has been more than 20 years since the discrepancy in the outcome between patients in CR with and without MRD has been demonstrated, and the field has come a long way from multiparameter flow cytometry to next-generation flow and next-generation sequencing, able to detect up to a limit of detection of a single myeloma cell from 1 million healthy counterparts. This review aims to summarize the current available data regarding MRD but also its potential future use as a coprimary outcome both in clinical and trial settings as a survival surrogate as well as its use to evaluate treatment efficacy and for adaptive response-based and early-rescue therapy. Furthermore, we discuss whether these concepts are applicable in different settings (eg, newly diagnosed and relapsed/refractory myeloma, patients who are eligible and ineligible for tansplant, and standard- and high-risk disease).
Introduction
Although complete response (CR) is achievable in patients with newly diagnosed multiple myeloma (NDMM), relapse remains inevitable, necessitating deeper and more sustainable responses to achieve an operational cure. Thus, the disease treatment must be driven beyond CR and beyond stringent CR toward molecular/flow CR to reach an eventual cure. The definition of CR is suboptimal, and the response to therapy is a key element in evaluating treatment efficacy. Twenty years ago, the use of multiparameter flow cytometry for the evaluation of measurable residual disease (MRD) was described, and patients in CR who had residual plasma cells (PCs) showed worse results than patients who did not (P = .02).1 Moreover, Puig et al used allele-specific oligonucleotide real-time quantitative polymerase chain reaction of immunoglobulin genes to assess for MRD in 103 patients with persistent disease and reported similar results, that is, patients with MRD-negative (MRD−) results did better than patients with MRD-positive (MRD+) results, and concluded that real-time quantitative polymerase chain reaction was a powerful technique to assess treatment efficacy and risk stratification in MM.2 In this article, we aim to summarize the available research evidence in the field of MRD use in MM and provide a few directions for future applications.
MRD as a surrogate end point for survival
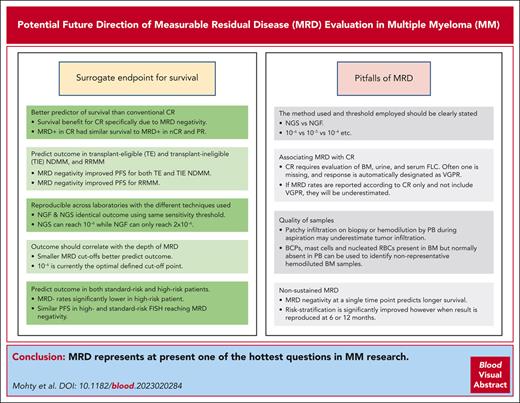
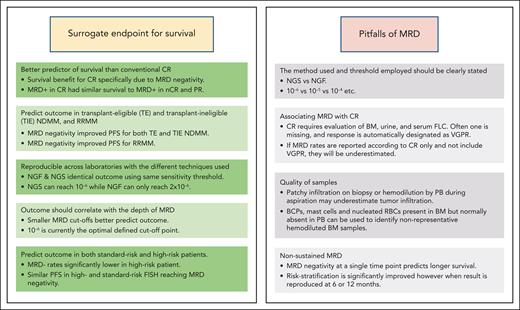
The question about the requirements for MRD to be a surrogate end point for survival and treatment monitoring is a crucial one. A meta-analysis conducted by Avet-Loiseau et al3 supported the claim that MRD status can be used as a surrogate for progression-free survival (PFS) in NDMM. In this endeavor, there are several barriers to overcome: (1) MRD must be a better predictor of survival than conventional CR, (2) MRD should predict the outcome not only in transplant-eligible (TE) and transplant-ineligible (TIE) NDMM but also in relapsed/refractory disease (RRMM), (3) results should be reproducible across laboratories with the different techniques used to measure the MRD, (4) the outcome should correlate with the depth of MRD, and (5) MRD should predict the outcome in both patients with standard risk and high risk (Figure 1).
The strengths and pitfalls of MRD. FLC, free light chains; nCR, near CR; RBC, red blood cell.
The strengths and pitfalls of MRD. FLC, free light chains; nCR, near CR; RBC, red blood cell.
With this background, Lahuerta et al4 compared the outcomes of patients in the Grupo Español de Mieloma 2000 (GEM 2000), GEM2005MENOS65 studies for TE MM, and the GEM2010MAS65 clinical trial for older patients with MM assessed for MRD 9 months after the study enrollment. Looking at conventional response, patients who achieved CR (n = 548 of 1230; 45%) experienced significantly superior PFS (median, 56 months) vs patients achieving near CR,5 partial response (PR), or <PR (median, 43, 33, and 20 months, respectively). However, on separating patients with MRD− results from those with MRD+ results, it was clear that the survival benefit for patients in CR was specifically due to MRD negativity (MRD− vs CR: P < .001). In fact, only MRD negativity conferred a significantly improved PFS (median, 63 months), because those with MRD+ results in CR had a similar survival to patients with MRD+ results in near CR and PR (median PFS, 27, 27 and 29 months, respectively), and for patients with MRD+ results achieving <PR, the median PFS was 11 months. Thus, MRD negativity proved to be superior at predicting the outcome compared with CR, although the MRD negativity threshold for detection was 10−4.
With respect to outcome prediction in patients with NDMM (TE and TIE) and RRMM, there are >100 publications and 4 meta-analyses supporting the association between MRD and PFS/overall survival (OS). An expanded meta-analysis by Munshi et al6 involving a large patient cohort of 8114 patients confirmed that MRD negativity has a positive effect on both PFS and OS. In fact, MRD negativity improved the PFS for both TE NDMM (hazard ratio [HR], 0.39; 95% confidence interval [CI], 0.32-0.46) and TIE NDMM (HR, 0.35; 95% CI, 0.29-0.42) as well as RRMM (HR, 0.30; 95% CI, 0.18-0.49). Across all 3 disease settings, patients with MRD− results did better. In young patients, the achievement of MRD negativity is indispensable to reach an operational cure. However, one needs to acknowledge that a small proportion of patients with detectable disease can achieve a plateau in their survival curve beyond 11 years and could be considered to have achieved an operational cure.7 These are likely the patients with a monoclonal gammopathy of undetermined significance (MGUS)-like gene signature in whom the survival benefit of achieving MRD negativity is less prominent compared with that in those with higher-risk disease.8 However, the latter group of patients is not easy to identify at the time of diagnosis.
Moreover, the attrition rate (especially in older and frail patients) is indeed very high after 2 or 3 lines of therapy. It is, therefore, very important to attempt to achieve the best possible response with earlier and frontline therapy.
From a technical standpoint, especially when it comes to reproducibility as a key requirement for MRD to be a surrogate for survival end points, experience with next-generation flow (NGF)-based and next-generation sequencing (NGS)-based methods of MRD assessment is accumulating. In particular, an important effort was recently made by the EuroFlow consortium to develop a highly sensitive, fully standardized approach for MRD detection using NGF in MM.9 Studies comparing NGS and NGF are summarized in the supplemental File, available on the Blood website. Although these studies show a correlation between NGS and NGF, additional studies using standardized methods are needed in order to know whether NGF may reliably replace the gold standard NGS in the future.
Depth of response is a key element to evaluate treatment efficacy and predict survival, and eradicating all tumor cells is necessary to cure most malignancies, which requires achieving and maintaining the deepest response possible. As mentioned earlier, CR in MM is a suboptimal definition because it relies on the low limit of detection techniques that are unable to distinguish residual tumor cells from polyclonal PCs. Using NGF, we can expect a limit of detection of ∼2 × 10−6, that is, detection of 2 tumor cells within 1 million healthy cells in almost 90% of patients with MM, and with the molecular gold standard NGS, we can reach sensitivities of 10−6. Other differences also exist in the individual features of the 2 techniques (cell viability, DNA extraction, time to obtain results, etc).10
For MRD status to qualify as a surrogate end point for the outcome (PFS and OS), the different thresholds of detection should affect the outcome regardless of the technique used. Avet-Loiseau et al11 used immunoglobulin gene NGS in a prospective trial to quantitate MRD and correlate this with PFS and OS. They showed that the best limit of detection was associated with the best discrimination in PFS and OS and suggested that 10−6 should be the optimal cutoff. Paiva et al12 looked at the feasibility, limit of detection, and clinical impact of NGF-based MRD assessment in the phase 3 PETHEMA/GEM2012 trial, and the 2 studies independently showed, using both assessment methods, that the lower the MRD levels, the better the outcome.
To be considered a surrogate end point for the outcome, MRD must also be able to predict survival outcomes for both patients with standard and high risk MM. The impact of MRD negativity in both patients with standard and high risk MM defined via fluorescence in situ hybridization (FISH) [ie, t(4;14), t(14;16), and/or del(17p)] was also investigated by Paiva et al.12 Even though MRD− rates were significantly inferior in patients with high- vs standard-risk FISH (37% vs 50%, respectively; P = .03), the 3-year PFS rates were similar between patients with high- and standard-risk FISH reaching MRD negativity (94% and 91%, respectively; P = .56); by contrast, MRD+ cases with high- and standard-risk FISH had a median PFS of 27 and 35 months, respectively (P = .025).12 In contrast, in the IFM 2009 trial, PFS in patients at high risk was inferior based on MRD results at the start of maintenance therapy, with an adjusted HR of 1.69 (95% Cl,1.14-2.48; P 5 .008).13 Importantly, there was no difference in the PFS of patients with high risk and standard risk achieving MRD negativity at the end of maintenance therapy, with an adjusted HR (covariates: MRD, treatment, and international staging system stage) of 1.08 (95% Cl,0.61-1.91; P = .785). This study indicates that patients with high risk still have an impaired prognosis even when they achieve MRD negativity, possibly through early loss of MRD negativity because sustained MRD negativity seems to overcome the deleterious impact of high-risk cytogenetics. Finally, high-risk cytogenetics are probably not all equal in terms of the impact of MRD negativity on patients' outcomes. In a retrospective study by Chakraborty et al, in the subgroups with deletion (17p) (n = 84) and those with ≥2 high-risk cytogenetic abnormalities (HRCAs) (n = 32), MRD negativity did not translate into a superior PFS or OS, whereas in patients with t(4;14) (n = 65), MRD negativity translated into significantly superior PFS and OS.14
In contrast, we must highlight that some patients had MRD+ results (limit of detection, 10−6) >5 years after remission and display a prevalence of normal vs abnormal monoclonal PC immune phenotype (MGUS-like).15
In summary, it is reasonable to advocate that, although all MRD negativity is not equal, MRD negativity is a new end point in MM therapy, and MRD negativity should be added to the criteria for response to treatment assessments to allow uniform reporting of results within or outside of clinical trials. In addition, although we acknowledge the specific case of the small proportion of patients with the MGUS-like gene signature in whom the survival benefit of achieving MRD negativity is less prominent, we believe MRD should become a new goal of MM treatment for most patients, and it should be included with PFS and OS as an end point in clinical trials. It has already been incorporated into the criteria for response assessment at the threshold of detection of 10−5 while being sustained for at least 1 year and is increasingly becoming a new objective in myeloma treatment. Continuous efforts should be made to include NGF or NGS in future trials to better define their optimal use before extending these methods to daily practice.16
MRD outside the bone marrow (BM)
MM displays a high spatial and temporal heterogeneity. In addition, there is a close relationship between baseline focal lesions identified via imaging techniques and relapse clones.17 The MM clonal architecture, which can include site-specific subclones, makes it difficult to capture the full extent of clonal diversity in MM. Thus, single-point BM sampling may not capture the tumor heterogeneity and is less accepted by patients when repeated for serial assessments. The so-called minimally invasive liquid biopsy allows for identifying and analyzing circulating MM cells, including possible detection of disease burden, molecular alterations, and monitoring treatment response. A major challenge in MM is detecting MRD in the peripheral blood (PB). To determine whether plasma could efficiently replace BM in MRD assessment using this molecular target, Mazzotti et al18 conducted a comparative prospective study, in which paired BM and PB samples were obtained from 37 patients during follow-up. MRD was assessed using deep sequencing. There were false-negative results in the PB of 44% of the patients (r = 0.003; P = .98). The study demonstrated the absence of a correlation between circulating tumor DNA (ctDNA) and the BM for MRD via NGS using only immunoglobulin gene rearrangements, suggesting that ctDNA alone is not an applicable routine marker of disease status in these conditions. Sanoja-Flores et al19 investigated the prognostic impact of ctPCs via NGF in the blood of 137 patients with NDMM after active treatment outside clinical trials. From the prognostic point of view, their results based on real-world MM cases show that the absence vs presence of blood ctPC, via NGF, is a powerful independent prognostic marker for PFS measured since the time of BM MRD/ctPC assessment among the entire MM cohort (HR, 5.1; 95% CI, 2.9-8.9; P < .0001). The median PFS for patients with PB ctPC-negative (n = 101) and PB ctPC-positive (n = 36) results was 46 and 9 months, respectively (P < .0001). Nevertheless, a significant proportion of patients who had BM MRD+ results still had undetectable ctPCs in (paired) blood samples: 55 of 137 (40%). These findings indicate that ctPC is a less sensitive MRD marker in MM than BM MRD and, therefore, not an applicable routine marker of disease status in these conditions.
Mass spectrometric (MS) methods represent attractive, minimally invasive alternatives to BM-based MRD evaluation. Major progress has been made in developing ultrasensitive MS methods. Both the clonotypic peptide approach and the intact protein approach make use of the unique mass and sequence of the M-protein to measure its concentration. Each method offers specific advantages, such as high sensitivity or high throughput.20 This could pave the way for more frequent MRD monitoring to allow for the detection of early disease relapse. Puig et al demonstrated that MS is more sensitive to detect the M-protein in patients with MM, both at baseline and during treatment, and provides a more accurate prediction of the patients' outcomes.21 Therefore, prospective studies evaluating MS along with BM MRD via NGS or NGF are indispensable to decipher the respective roles of each approach in the future and to know whether it may be possible to routinely use MS on PB samples to detect MRD instead of using the more invasive BM approach, at least at some time points.
Imaging also plays an important role in MM MRD evaluation. The role of imaging is discussed in the supplemental File. Overall, additional studies are needed to rule out the respective roles of imaging and biology for MRD assessment. If imaging allows for the detection of extramedullary disease, it remains to be established whether it is well corelated with NGS and NGF for evaluation of intramedullary disease, making both approaches more complementary than exclusive.
Pitfalls of MRD
In any MRD analysis, whether in clinical practice or studies, potential pitfalls should be considered pertaining to evaluation and particularly reporting (Figure 1).
The first pitfall is associating MRD with CR, which can be illustrated by looking at 2 studies: CASSIOPEIA and GMMGHD7. The CR rate vs MRD negativity rate (10−5) after consolidation in CASSIOPEIA was 39% vs 64%, respectively, and in the GMMGHD7, the rate after induction was 24% vs 50% (10−5), respectively. At both time points, MRD rates were much higher than CR rates. The problem is that CR requires the evaluation of the BM, the urine, and the serum-free light chains, but as soon as one of these values is missing, the response is not designated as CR but as very good PR. If MRD rates are reported according to CR, they will be underestimated, which will lead to confusion. A study by Puig et al22 assessed the first 164 patients after consolidation, treated in the PETHEMA/GEM2021MENOS65 trial. They compared the predictive value of the 3 techniques: immunofixation electrophoresis, MS coupled with liquid chromatography, and NGF. Surprisingly, the presence or absence of disease via immunofixation electrophoresis did not discriminate patients with a different PFS (P = .2923), but both MS and NGF segregated 2 cohorts with a significantly different PFS (HR, 2.18; 95% CI, 1.27-3.75; P = .0016 and HR, 2.83; 95% CI, 1.69-4.72; P < .0001, respectively). The median PFS was 4.4 months in the patients who had MS-positive and NGF-positive results and was not reached in the patients who had MS- and NGF-negative results. This raised the issue of the conflicting results between the achievement of CR and MRD negativity. Indeed, despite the US Food and Drug Administration’s 2020 guidline that MRD should be assessed only in patients who are in CR, assessment of CR via immunofixation electrophoresis may be misled by the long half-life of M-protein, therapeutic antibodies interfering, or inaccurate assessment of CR (missing assessment of BM, urine, or serum-free light chains). Therefore, the discrepancy between the assessment of CR and MRD negativity in those patients suggests that MRD should also be assessed in patients achieving very good PR.23
In addition, to avoid any additional pitfalls in data interpretation, particular attention should be paid when reporting MRD analysis, whether in clinical practice or trials. The method used (NGS/NGF) and, importantly, the threshold of detection used (as previously mentioned, a limit of detection of 10−6 is very different from 10−4) should be clearly stated.
Another critical issue is linked to the quality of BM samples (which affects both NGS and NGF). Unfortunately, the presence of patchy infiltration in the PB upon biopsy or hemodilution of PB during aspiration may underestimate tumor infiltration.24 B-cell precursors (BCPs), mast cells, and nucleated red blood cells that are present in the BM but normally absent in the PB can be used as a sign of hemodilution to identify nonrepresentative BM samples. Using flow cytometry, it is possible to simultaneously assess MRD and hemodilution to avoid potential false-negative MRDs. Hemodiluted BM aspirate samples do not qualify for MRD assessment. PFS and OS of 118 patients with TIE MM enrolled in the PETHEMA/GEM2010MAS65 clinical trial were stratified according to detectable vs undetectable MRD at the end of treatment and, within the latter, according to the percentage of BM BCPs below or above the median value (0.31%) observed in healthy adults. In patients with undetectable MRD, median PFS was significantly higher in those with BCPs ≥.31% compared with <0.31% (52 vs 28 months; P = .033). Three-year OS rates were 95.5% vs 74%, respectively (P = .048).
The last pitfall to mention is nonsustained MRD. Patients achieving MRD negativity and displaying transient or nonsustained MRD negativity may present a bad prognostic group. It has become evident that although MRD negativity at a single time point clearly predicts longer survival, risk stratification is significantly improved when this result is reproduced at 6 or 12 months. Among patients achieving at least CR, if patients who have MRD− results with sustained negativity for ≥12 months are separated from those without, the former group has a better PFS. This was clearly shown in a recent study of sustained MRD negativity (NGS threshold of detection, 10−5) in NDMM and the impact of daratumumab in the MAIA and ALCYONE trials.25
MRD in clinical practice and to make treatment decisions
The evidence that supports MRD assessment in clinical practice and in making treatment decisions has several areas to focus on, based on data from current clinical trials: evaluating treatment efficacy and comparing 2 treatment approaches; adapting therapy according to the MRD follow-up; adapting maintenance intensity and duration; and introducing early-rescue intervention (ERI; Table 1).
Use of MRD in clinical practice and to make treatment decisions
| Study . | Number of patients and characteristics . | Chemotherapy . | MRD technique and limit of detection . | MRD-related outcomes . |
|---|---|---|---|---|
| MRD for evaluating treatment efficacy | ||||
| PETHEMA/GEM2012 trial randomized phase 3 study NCT01916252 Paiva et al26 | N = 458 TE NDMM | Induction VRd (×6) ASCT Consolidation VRd (×2) | NGF median, 3 × 10−6 | MRD– status after induction, 28%
|
| UK OPTIMUM /MUKnine trial phase 2 study NCT03188172 Kaiser et al27 | N = 95 ultrahigh-risk NDMM | Induction D-CVRd (×6) ASCT Consolidation D-CVRd (×6) D-VR (×12) Maintenance D-R | MFC, 10−5 | MRD status on day +100 after ASCT: MRD–, 64% MRD+, 14% MRD not evaluable, 22% |
| GMMG-HD7 study randomized phase - study NCT03617731 Goldschmidt et al28 | N = 662 TE NDMM | Induction: VRd ± I | NGF, 10−5 | After induction: MRD– status:
|
| MRD for comparing the efficacy of different treatment approaches | ||||
| CASSIOPEIA randomized phase 3 study NCT02541383 Moreau et al29 | N = 1085 TE NDMM | Induction D-VTd vs VTd (×4) ASCT Consolidation VTd vs VTd (×2) Maintenance D vs observation | MFC, 10−5 | Day +100 after ASCT: MRD– status:
|
| BENEFIT: IFM2020-05 randomized phase 3 study NCT04751877 Leleu et al30 | N = 270 TIE, nonfrail NDMM | Isa-VRD vs Isa-Rd | NGS, 10−5 | Primary end point: MRD at 18 mo (ongoing) |
| EMN28/CARTITUDE-6 randomized phase 3 study NCT0525708331 | N = 750 TE NDMM | cilta-cel vs ASCT | NGS, 10−5 | Dual primary end points: PFS and sustained MRD |
| MRD for adapting therapy | ||||
| MIDAS randomized phase 3 study NCT04934475 Moreau32 | N = 716 TE NDMM | Induction Isa-KRd (×6) Standard risk (NGS, <10−5): arm A; 6 additional cycles of Isa-KRd vs arm B: ASCT followed by 2 cycles of Isa-KRd; High risk (NGS, >10−5): arm C: ASCT followed by 2 cycles of Isa-KRd vs arm D: tandem ASCT | NGS, 10−5 | Randomization based on MRD assessment after induction |
| MRD for adapting maintenance therapy and duration | ||||
| EMN017/MMY3014 (Perseus) randomized phase 3 NCT0371060333 | N = 690 TE NDMM | Induction: D-VRd vs VRd (×4) ASCT Induction: D-VRd vs VRd (×2) Maintenance DR vs R until progression | NGS, 10−5 | D-VRD arm : 12 mo sustained MRD and ≥ 2 y maintenance: D interruption |
| GEM2014 trial randomized phase 3 study NCT02406144 Rosinol et al34 | N = 316 TE NDMM | Maintenance ixazomib-Rd vs Rd for 2 y | NGF, 3 × 10−6 | At 2 y. MRD– status: maintenance interruption MRD+ status: pursue Rd alone for 3 more years |
| MRD for introduction of ERI | ||||
| REMNANT trial randomized phase 2/3 study NCT04513639 Askeland et al35 | N = 176 TE NDMM | Induction VRd (×4) ASCT (×1 or 2) Consolidation VRd (×2) | NGF, 10−5 | For patients with MRD– status: randomization
|
| GEM-TECTAL trial phase 2 study NCT0584961036 | N = 30: high risk, R-ISS-III, TE, and fit TIE NDMM | Induction D-VRd (×4) Intensification: D-teclistamab (×6) | NGF, 10−6 | MRD assessment after intensification: MRD– status: D-teclistamab 2y. MRD+ status or not in CR: D – talquetamab, and after 6 cycles: MRD– status, pursue for 2 y; MRD+ status, ASCT |
| Study . | Number of patients and characteristics . | Chemotherapy . | MRD technique and limit of detection . | MRD-related outcomes . |
|---|---|---|---|---|
| MRD for evaluating treatment efficacy | ||||
| PETHEMA/GEM2012 trial randomized phase 3 study NCT01916252 Paiva et al26 | N = 458 TE NDMM | Induction VRd (×6) ASCT Consolidation VRd (×2) | NGF median, 3 × 10−6 | MRD– status after induction, 28%
|
| UK OPTIMUM /MUKnine trial phase 2 study NCT03188172 Kaiser et al27 | N = 95 ultrahigh-risk NDMM | Induction D-CVRd (×6) ASCT Consolidation D-CVRd (×6) D-VR (×12) Maintenance D-R | MFC, 10−5 | MRD status on day +100 after ASCT: MRD–, 64% MRD+, 14% MRD not evaluable, 22% |
| GMMG-HD7 study randomized phase - study NCT03617731 Goldschmidt et al28 | N = 662 TE NDMM | Induction: VRd ± I | NGF, 10−5 | After induction: MRD– status:
|
| MRD for comparing the efficacy of different treatment approaches | ||||
| CASSIOPEIA randomized phase 3 study NCT02541383 Moreau et al29 | N = 1085 TE NDMM | Induction D-VTd vs VTd (×4) ASCT Consolidation VTd vs VTd (×2) Maintenance D vs observation | MFC, 10−5 | Day +100 after ASCT: MRD– status:
|
| BENEFIT: IFM2020-05 randomized phase 3 study NCT04751877 Leleu et al30 | N = 270 TIE, nonfrail NDMM | Isa-VRD vs Isa-Rd | NGS, 10−5 | Primary end point: MRD at 18 mo (ongoing) |
| EMN28/CARTITUDE-6 randomized phase 3 study NCT0525708331 | N = 750 TE NDMM | cilta-cel vs ASCT | NGS, 10−5 | Dual primary end points: PFS and sustained MRD |
| MRD for adapting therapy | ||||
| MIDAS randomized phase 3 study NCT04934475 Moreau32 | N = 716 TE NDMM | Induction Isa-KRd (×6) Standard risk (NGS, <10−5): arm A; 6 additional cycles of Isa-KRd vs arm B: ASCT followed by 2 cycles of Isa-KRd; High risk (NGS, >10−5): arm C: ASCT followed by 2 cycles of Isa-KRd vs arm D: tandem ASCT | NGS, 10−5 | Randomization based on MRD assessment after induction |
| MRD for adapting maintenance therapy and duration | ||||
| EMN017/MMY3014 (Perseus) randomized phase 3 NCT0371060333 | N = 690 TE NDMM | Induction: D-VRd vs VRd (×4) ASCT Induction: D-VRd vs VRd (×2) Maintenance DR vs R until progression | NGS, 10−5 | D-VRD arm : 12 mo sustained MRD and ≥ 2 y maintenance: D interruption |
| GEM2014 trial randomized phase 3 study NCT02406144 Rosinol et al34 | N = 316 TE NDMM | Maintenance ixazomib-Rd vs Rd for 2 y | NGF, 3 × 10−6 | At 2 y. MRD– status: maintenance interruption MRD+ status: pursue Rd alone for 3 more years |
| MRD for introduction of ERI | ||||
| REMNANT trial randomized phase 2/3 study NCT04513639 Askeland et al35 | N = 176 TE NDMM | Induction VRd (×4) ASCT (×1 or 2) Consolidation VRd (×2) | NGF, 10−5 | For patients with MRD– status: randomization
|
| GEM-TECTAL trial phase 2 study NCT0584961036 | N = 30: high risk, R-ISS-III, TE, and fit TIE NDMM | Induction D-VRd (×4) Intensification: D-teclistamab (×6) | NGF, 10−6 | MRD assessment after intensification: MRD– status: D-teclistamab 2y. MRD+ status or not in CR: D – talquetamab, and after 6 cycles: MRD– status, pursue for 2 y; MRD+ status, ASCT |
C, cyclophosphamide; cilta-cel, ciltacabtagene autoleucel; d, dexamethasone; D, daratumumab; I, isatuximab; MFC, multiparameter flow cytometry; OR, odds ratio; R, lenalidomide; TE, transplantation eligible; TIE, transplantation ineligible; V, bortezomib.
MRD for evaluating treatment efficacy
The value of MRD in evaluating treatment efficacy is illustrated by Paiva et al in the PETHEMA/GEM2012 trial, which included the 3 phases of the triplet therapy: bortezomib-Rd (VRd) induction (6 cycles), autologous stem cell transplantation (ASCT), and VRd consolidation (2 cycles).12 MRD was used to evaluate the efficacy of each phase. MRD negativity after induction was observed in 28% (n = 129), after ASCT in 42% (n = 194), and after consolidation in 45% (n = 208).26 However, patients were grouped according to the following MRD logarithmic levels: (1) ≥10−4, (2) ≥10−5 to <10−4, and (3) ≥2 × 10−6 to 10−5, and the rate of patients in the MRD logarithmic level 3 increased from 9% after ASCT to 19% after consolidation. This could justify an additional 2 cycles of consolidation in patients in whom MRD decreases by 2 logs after 2 cycles of VRd consolidation until it is undetectable before going into maintenance, which would sustain the response.
The UK OPTIMUM/MUKnine trial evaluated MRD status after ASCT and PFS among 107 patients with ultrahigh-risk NDMM ([≥2 high risk lesions: t(4;14), t(14;16), t(14;20), gain(1q), del(1p), del(17p)] or gene expression SKY92 (SkylineDx) profiling or with PCL]. Patients were treated with daratumumab-CVRd (D-CVRd) induction (6 cycles), augmented high-dose melphalan, and ASCT, followed by D-VRd consolidation 1 for 6 cycles, D-VR consolidation 2 for 12 cycles, and D-R maintenance until progression. BM aspirates suitable for MRD assessment via flow cytometry (10−5) were available for 81% of patients at the end of induction and 78% on day +100 after ASCT, demonstrating the feasibility of conducting a study with an end point based on centralized MRD status.27 MRD status was negative in 64% of patients, positive in 14% of patients, and not evaluable in 22% of patients on day +100 after ASCT.
Achievement of MRD negativity was also used as a primary end point in the GMMG-HD7 study that randomly assigned 660 patients to receive lenalidomide, bortezomib, and dexamethasone with or without isatuximab (Isa) as induction therapy in those with newly diagnosed MM who were TE.28 MRD was assessed at the end of induction chemotherapy with NGF (EuroFlow). MRD negativity after induction therapy was achieved in 166 (50%) patients in the Isa group vs 117 (36%) in the control group (odds ratio, 1·82 [95% CI 1·33-2·48]; P = 0·00017).
The best way to overcome high-risk cytogenetics is through the achievement of MRD negativity, which was shown by Paiva et al12 in 2019. In the GEM2012 trial, outcomes were progressively poorer for patients with revised international staging system (R-ISS)-I, R-ISS-II, and R-ISS-III status when MRD status remained positive (36-month PFS rates of 62%, 53%, and 28%, respectively). By contrast, there were no significant differences in the 36-month PFS rate for patients who had MRD− results (95%, 94%, and 88%, respectively). Thus, patients with R-ISS-III status overcame a poor prognosis through the achievement of undetectable MRD, which should have been the goal in patients who were at high risk. However, despite the value of MRD achievement toward overcoming poor prognosis, one should bear in mind that patients with HR MM are unlikely to achieve sustained deep MRD negativity, highlighting the challenges posed by this population.
MRD for comparing the efficacy of different treatment approaches
The value of MRD in comparing the efficacy of 2 treatment approaches can be shown by recent trials such as CASSIOPEIA, a phase 3 study comparing D-V-thalidomide-d (D-VTd) with VTd before and after ASCT in 1085 TE NDMM patients aged from 18 to 65 years from 111 sites (from September 2015 to August 2017). MRD via NGS and NGF was assessed throughout the trial: induction (4 cycles), consolidation (2 cycles), and maintenance. A second randomization was carried out to D monotherapy or observation after consolidation in patients with ≥PR.29
The ongoing clinical trial (BENEFIT: IFM2020-05) for nonfrail patients with TIE NDMM aged from 65 to 79 years, randomly assigned to either Isa-VRD or Isa-Rd, is using MRD negativity (NGS 10−5) after 18 months as the primary end point.30
Results are also eagerly awaited from the EMN28 phase 3 randomized trial of ciltacabtagene autoleucel vs ASCT in 750 patients with TE NDMM aged ≥18 years, stratified based on the ISS stage, cytogenetic risk, and age. The dual primary end points are PFS and sustained MRD negativity in patients with CR (NGS 10−5).31 The value of conventional treatment with D-VRd + ASCT vs D-VRd + ciltacabtagene autoleucel will be compared.
MRD for adapting therapy
MRD assessment may be also of great value in adapting therapy according to the MRD follow-up. In the IFM MIDAS (MRD Adapted Strategy) trial, in which all patients with TE aged from 18 to 65 years received Isa-KRd induction (6 cycles) and were then stratified based on standard risk or high risk, not via cytogenetics but depending on MRD status, standard-risk NGS had a threshold of <10−5, and high-risk NGS had a threshold of >10−5. Patients were then randomly assigned, depending on MRD status, to 1 of 4 treatment arms as follows: standard risk (NGS, <10−5): arms A and B, arm A: 6 additional cycles of Isa-KRd vs arm B: ASCT followed by 2 cycles of Isa-KRd; and high risk (NGS, >10−5): arms C and D, arm C: ASCT followed by 2 cycles of Isa-KRd vs arm D: tandem ASCT. For maintenance (3 years), patients in arms A/B would receive R, and in arms C/D, patients would receive a more intensive treatment (iberdomide and Isa).32 Finally, the UK group started a similar adapted therapy phase 2 trial, the RADAR trial, to define the role of predictive biomarkers.37 Patients with TE rendered with MRD−results through induction/ASCT are being studied to define whether a de-escalation of posttransplant therapy is safe and effective, whereas those with MRD+ results even after ASCT are being studied for the impact of treatment escalation, including immunotherapy. Such a dynamic assessment of the risk during the course of therapy represents an appealing approach for the most accurate risk evaluation when combined with other baseline risk parameters. Nevertheless, we must point out that such approaches should, in the future, try to incorporate sustained MRD rather than MRD at a single point because patients with transient or nonsustained MRD negativity may represent a bad prognosis group.
MRD for adapting maintenance therapy and duration
The fourth strategy to support the use of MRD in clinical practice is to adapt maintenance intensity and duration. The latter would bring significant added value in terms of potential cost-reduction but also in minimizing short- and long-term adverse events. In the ongoing aforementioned RADAR trial, the choice of maintenance therapy was based on MRD status posttransplant and cytogenetics risk, with reassessment of MRD after maintenance dictating whether therapy should be continued. In the MASTER trial, patients were classified according to HRCAs defined based on gain/amp 1q, t(4;14), t(14;16), t(14;20) or del(17p). Among 123 participants, 57% were at high risk. Eighty-four patients reached MRD-SURE (treatment-free observation and MRD surveillance), comprising 62%, 78%, and 63% with 0, 1, and ≥2 HRCAs (ultrahigh risk), respectively). The 2-year PFS was 87% (91%, 97%, and 58% for patients with 0, 1, and ≥2 HRCAs, respectively; P < .001). The cumulative incidence of MRD resurgence or progression 12 months after treatment cessation was 4%, 0%, and 27% for patients with 0, 1, or ≥2 HRCAs, respectively. Results for OS were in a similar direction (P = .03). For patients with 0 or 1 HRCA, there is the opportunity to use MRD surveillance instead of indefinite maintenance, but treatment should not be stopped in patients who were at ultrahigh risk because they will relapse quickly.38 The limit of detection should be at least 10−6 in this patient population, otherwise residual cells will be missed, as was shown in the patients with poor PFS at R-ISS-III.
Another possibility for adaptive maintenance therapy intensity was proposed in the EMN017/MMY3014 phase 3 registration trial (Perseus), which enrolled 640 patients with NDMM aged from 18 to 70 years. It compared D-VRd vs VRd with induction (4 cycles); ASCT, followed by consolidation (2 cycles); and DR or R maintenance until progression in the D-VRd and VRd groups, respectively. The primary end point was PFS, and the secondary end point was MRD after consolidation (NGS 10−5). In the D-VRd group, patients with 12 months of sustained MRD negativity after at least 2 years of maintenance were taken off D, which was resumed upon MRD conversion.33
The Spanish GEM2014 trial also investigated the role of MRD in optimizing maintenance. After induction and consolidation, stable patients were randomly assigned to maintenance treatment with ixazomib-Rd (IRd) vs Rd for 2 years. After 2 years, similar to the EMN study, patients who had MRD− results (NGS, 3 × 10−6) discontinued maintenance, and those who had MRD+ results continued with Rd for 3 additional years. The PFS at 2 years was significantly different (P < .001), with those who had MRD− results, continuing to do well with very few relapses, whereas those who had MRD+ results despite having maintenance, continuously relapsed.34
MRD for introduction of ERI
This highlights the importance and need for the introduction of ERI according to the MRD status. Treating MRD relapse has not been evaluated in a randomized fashion, but the REMNANT study, a Norwegian randomized phase 2/3 study of 391 patients with TE, is evaluating whether treating MRD relapse after first-line treatment prolongs the PFS and OS for patients with MM compared with treating relapse after first-line treatment at disease progression. Patients are enrolled at diagnosis and receive VRd induction (4 cycles), single or tandem ASCT, and VRd consolidation (4 cycles). Patients who have MRD− results(NGF 10−5) are randomly assigned to either treatment at MRD reappearance or disease progression based on conventional International Myeloma Working Group (IMWG) criteria. The primary end point of the phase 2 part of the study is MRD− (NGF, 10−5) CR 30 or 45 days after consolidation.39 To date, 26 patients have been evaluated, and 13 patients (50%) were in ≥CR, had MRD− results, and have been enrolled in part 2 of the study.35
The Spanish group is also designing the GEM-TECTAL trial for candidates with TE and fit candidates with TIE with high-risk FISH chromosome abnormalities [del(17p), t(4;14), t(14;16), and/or 1q amplification], and classified as being at R-ISS-III.36 After 4 cycles of D-VRd, MRD evaluation, and stem cell collection, they will receive 6 cycles of the bispecific T-cell engager teclistamab. If they then have MRD− results, they will continue receiving teclistamab for 2 years, and if MRD+ results, they will have 6 cycles of another bispecific antibody, namely talquetamab (plus D). Patients who have MRD− results will stay on this therapy for 2 years. Patients who have MRD+ results will proceed to receive ASCT.
Concluding remarks
In conclusion, MRD currently represents one of the hottest questions in MM research. Defining the requirements for MRD to be a surrogate end point for survival and for treatment monitoring is a critical endeavor. The most sensitive and less invasive techniques need to be implemented to evaluate treatment efficacy, particularly if complete eradication of tumor cells is the objective. The latter is particularly true in the frontline setting, in which the best possible treatment should be offered to further improve the outcome in patients.
Acknowledgments
The authors acknowledge the support and work of the Integroupe Francophone du Myélome (IFM). The authors thank patients and families for their contribution to the different myeloma and IFM research protocols. M.M. thanks Junia Melo (University of Adelaide, Australia) for the critical reading of the manuscript.
Authorship
Contribution: M.M., H.A.-L., and J.-L.H. performed the bibliographic search and wrote the first version of the manuscript; F.M. performed the bibliographic search and revised the manuscript; and all authors contributed to design and writing, editing, and revising this manuscript.
Conflict-of-interest disclosure: M.M. has received research support and lecture honoraria from Adaptive, Amgen, Astellas, BMS, GlaxoSmithKline, Janssen, Jazz, Novartis, Pfizer, Takeda, Sanofi, and Stemline, all outside the scope of this work. F.M. reports honoraria from Therakos/Mallinckrodt, Janssen, Sanofi, JAZZ Pharmaceuticals, Gilead, Novartis, and Astellas, all outside the scope of this work. The remaining authors declare no competing financial interests.
Correspondence: Mohamad Mohty, Service d’Hématologie Clinique et Thérapie Cellulaire, AP-HP, Hôpital Saint-Antoine, 27, rue Chaligny, 75012 Paris, France; e-mail: mohamad.mohty@inserm.fr.
References
Author notes
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal