Key Points
CS585 is a novel prostacyclin receptor agonist that effectively inhibits integrin activation, granule secretion, and aggregation.
CS585 is a highly potent and selective compound available both orally and IV and prevents thrombosis between 24 and 48 hours.
Abstract
Cardiovascular disease remains the primary cause of morbidity and mortality globally. Platelet activation is critical for maintaining hemostasis and preventing the leakage of blood cells from the vessel. There has been a paucity in the development of new drugs to target platelet reactivity. Recently, the oxylipin 12(S)-hydroxy-eicosatrienoic acid (12-HETrE), which is produced in platelets, was shown to limit platelet reactivity by activating the prostacyclin receptor. Here, we demonstrated the synthesis of a novel analog of 12-HETrE, known as CS585. Human blood and mouse models of hemostasis and thrombosis were assessed for the ability of CS585 to attenuate platelet activation and thrombosis without increasing the risk of bleeding. Human platelet activation was assessed using aggregometry, flow cytometry, western blot analysis, total thrombus formation analysis system, microfluidic perfusion chamber, and thromboelastography. Hemostasis, thrombosis, and bleeding assays were performed in mice. CS585 was shown to potently target the prostacyclin receptor on the human platelet, resulting in a highly selective and effective mechanism for the prevention of platelet activation. Furthermore, CS585 was shown to inhibit platelet function in human whole blood ex vivo, prevent thrombosis in both small and large vessels in mouse models, and exhibit long-lasting prevention of clot formation. Finally, CS585 was not observed to perturb coagulation or increase the risk of bleeding in the mouse model. Hence, CS585 represents a new validated target for the treatment of thrombotic diseases without the risk of bleeding or off-target activation observed with other prostaglandin receptor agonists.
Introduction
To date, the pharmacological regulation of platelet function in clinical practice has been limited to targeting cyclooxygenase-1, phosphodiesterase, integrin receptor αIIbβ3, and purinergic receptor P2Y12.1 However, although preclinical studies have identified several other receptors and enzymes involved in platelet regulation, none have successfully been able to receive regulatory approval for clinical treatment.2 The limitation in advancement often lies in the potential for inhibition of platelet function, resulting in increased bleeding, instability, or off-target effects.1-5 To address these challenges, we took advantage of an endogenous regulator of platelet function that targets the prostacyclin (IP) receptor. Recently, it was shown that platelets form the oxylipin 12(S)-hydroxy-eicosatrienoic acid (12-HETrE) through oxidation of the fatty acid dihomo-γ-linolenic acid by the enzyme 12-lipoxygenase.6 12-HETrE was shown to have a high affinity for the IP receptor, increase cyclic adenosine monophosphate (cAMP) formation in the platelet, activate protein kinase A (PKA), and subsequently attenuate platelet activation.7,8
CS585, a newly synthesized analog of 12-HETrE, is an orally available, potent, selective, and stable IP receptor agonist drug candidate that inhibits platelet activation. Herein, we demonstrated pharmacologically and genetically that CS585 regulates human platelet function through activation of the IP receptor. The selectivity and potency of CS585 make this compound a promising candidate for targeting the IP receptor. Its stability in the blood gives CS585 the potential to be used as an antiplatelet drug for the prevention of thrombosis without impacting coagulation or bleeding.
Material and methods
A full description of the methods is available in supplemental Materials, available on the Blood website.
Synthesis of CS585
The synthesis of CS585 involved a 3-step process starting with a pyrazine derivative, resulting in the final product 5-((4-((5,6-diphenylpyrazin-yl)(isopropyl)amino)butyl)thio)-2,4-dihydro-3H-,12,4-triazol-3-one (CS585).
In vivo pharmacokinetics of CS585
CD1 wild-type (WT) mice received 6 mg/kg CS585 IV or per oral administration. CS585 plasma concentration was monitored by LC/MS/MS.
Human blood collection and platelet isolation
Research involving human subjects was carried out in accordance with the Declaration of Helsinki. Informed consent was obtained before enrollment in the study, with the approval of the University of Michigan Institutional Review Board (HUM00197665). Platelet-rich plasma (PRP) and washed platelets were isolated and suspended at 3 × 108 platelets per mL, as previously described.7,9
Isolation of mouse platelets
Platelet aggregation
Integrin αIIbβ3 activation and granule secretion
Washed human platelets were treated with the vehicle, CS585, or 12-HETrE. Platelets were stained with antibodies αIIbβ3, P-selectin, and CD63 and stimulated with convulxin. The fluorescence intensity was measured using flow cytometry (CytoFLEX, Beckman Coulter).
Vasodilator-stimulated phosphoprotein phosphorylation assay
Washed human and mouse platelets were incubated with vehicle, CS585, 12-HETrE, or forskolin for 10 minutes. The samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted for total vasodilator-stimulated phosphoprotein (VASP), phosphorylated VASP,8,9 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
cAMP and cGMP assays
Washed human platelets were treated with 3-isobutyl-1-methylxanthine for 30 minutes before treatment with vehicle, CS585, forskolin, or PAPA NONOate for 1 minute. The reactions were quenched and cAMP and cyclic guanosine monophosphate (cGMP) detection assays (Enzo Life Sciences) were performed as previously described.10
Phosphatidylserine exposure
Washed human platelets were treated with vehicle, CS585, or iloprost for 10 minutes. Male and female WT mice were IV injected with either vehicle or CS585. After 4 hours, the washed platelets were incubated with antibodies for annexin and P-selectin, recalcified with 2 mM CaCl2 and stimulated with an agonist.12 The fluorescence intensity was measured using flow cytometry (CytoFLEX, Beckman Coulter).
Total thrombus formation analysis system
Ex vivo microfluidic perfusion flow chamber
Microfluidic perfusion chambers (μ-Slide VI 0.1; ibidi) were coated with 100 μg/mL type I collagen (Chrono-log). Citrated whole blood was incubated with vehicle, CS585, or iloprost. Platelets were fluorescently labeled with 2 μM 3,3′-dihexyloxacarbocyanine iodide (DiOC6) (Thermo Fisher Scientific). Blood was recalcified with 5 mM CaCl2 and immediately perfused at arterial shear (1800 s−1).9,15
Laser ablation cremaster arteriole thrombosis model
Male WT mice (8-12 weeks of age) were anesthetized7,15 and treated (vehicle or CS585) via IV or oral administration before the induction of vascular injury using a laser ablation system (Ablate! Photoablation system; 3i). Images were acquired in real-time, using a 63× water-immersion objective, as previously described,16,17 and analyzed using Slidebook 6.0.
FeCl3-induced carotid artery thrombosis assay
Mouse platelets were isolated from genotype-matched donor mice, as previously described.7 Male and female mice were anesthetized and IV injected with fluorescently labeled platelets, followed by treatment with vehicle or CS585. Carotid artery injury was induced by topical application of 10% FeCl3. Platelet adhesion and thrombus growth were recorded under a 5× air objective using a Zeiss Axio Examiner Z1 fluorescence microscope. Vessel occlusion was defined as the formation of an occlusive thrombus.7,18
Thromboelastography
Tail bleeding assay
Statistics analysis
Data analysis was performed using GraphPad Prism 9 software (GraphPad Software). Data are represented as mean ± standard error of the mean (SEM). The statistical tests used for the individual experiments are listed in the corresponding figure legends.
Results
Synthesis of CS585
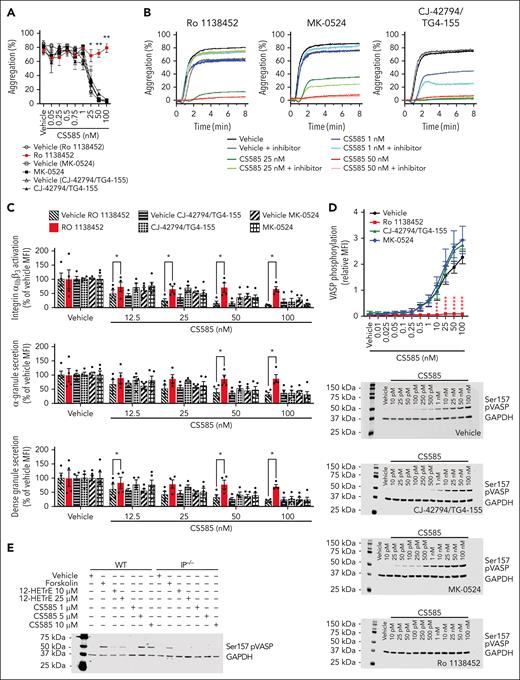
12-HETrE was recently shown by our research group to inhibit platelet activation by selectively activating the IP receptor on the platelet, resulting in activation of PKA.6-8 This signaling pathway results in the significant attenuation of platelet activation, including integrin αIIbβ3 activation, granule secretion, and further clot formation.8,22 Using the structure of 12-HETrE as a starting point, a novel analog 4-((5-diphenylpyrazin-2-yl)(isopropyl)amino)butan-1-ol (3) was developed, which functions in a similar manner to the parent compound, and was named CS585 (Figure 1A). The synthesis pathway of CS585 is shown in Figure 1B. To determine the stability of CS585, the compound was resuspended in vehicle and stored at 37°C for 21 days. An aliquot was assessed daily by mass spectrometry for stability. CS585 was found to be stable throughout the entire 21-day assessment (supplemental Table 1). To determine the half-life of CS585 in vivo as well as oral availability, mice were dosed with CS585 by mouth via oral gavage, followed by determination of pharmacokinetics (PK) in the blood for 24 hours. After oral administration, CS585 was detected in the blood within 15 minutes and observed to have a half-life of 4 hours (Figure 1C). To compare the potency of CS585 with that of 12-HETrE, washed human platelets were incubated with increasing concentrations of CS585 or 12-HETrE. After stimulation with 0.5 μg/mL collagen, platelet aggregation was assessed. Complete inhibition of platelet aggregation was observed with 100 nM CS585, whereas 5000 nM 12-HETrE was required to fully attenuate aggregation (Figure 1D). Finally, the effects of CS585 and 12-HETrE on markers of platelet activation were assessed by flow cytometry. A significant decrease in platelet activation (determined by the levels of active integrin αIIbβ3, P-selectin expression, and CD63 expression) was observed (Figure 1E).
Structure and kinetics of CS585. Potency of CS585 and 12-HETrE. (A) Structure of CS585. (B) Synthesis of CS585 involved a 3-step synthesis starting with a pyrazine derivative. The pyrazine derivative reacted with an alcohol to form 4-((5-diphenylpyrazin-2-yl)(isopropyl)amino)butan-1-ol. This intermediate was converted to a second intermediate, 4-((5-diphenylpyrazin-2-yl)(amino)butyl 4-methanebenzenesulfonate. Finally, the prostacyclin receptor agonist CS585 (5-((4-((5,6-diphenylpyrazin-yl)(isopropyl)amino)butyl)thio)-2,4-dihydro-3H-,12,4-triazol-3-one) is formed. (C) PK were assessed in mouse plasma over a 24-hour period. (D) Aggregation dose-response comparison of washed human platelets treated with vehicle, CS585 (3.125-100 nM), or 12-HETrE (500-15 000 nM), stimulated with collagen (0.5 μg/mL) (n = 4). (E) Integrin αIIbβ3 activation, α-granule, and dense granule secretion dose-response comparison of washed human platelets treated with vehicle, CS585 (3.125-100 nM) or 12-HETrE (500-15 000 nM) stimulated with convulxin (25 ng/mL) (n = 4). Data represent mean ± SEM. A one-way analysis of variance (ANOVA) was performed using an uncorrected Fisher least significant difference post hoc test. Asterisks denote statistical differences between the vehicle and treated groups: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. MFI, mean fluorescence intensity.
Structure and kinetics of CS585. Potency of CS585 and 12-HETrE. (A) Structure of CS585. (B) Synthesis of CS585 involved a 3-step synthesis starting with a pyrazine derivative. The pyrazine derivative reacted with an alcohol to form 4-((5-diphenylpyrazin-2-yl)(isopropyl)amino)butan-1-ol. This intermediate was converted to a second intermediate, 4-((5-diphenylpyrazin-2-yl)(amino)butyl 4-methanebenzenesulfonate. Finally, the prostacyclin receptor agonist CS585 (5-((4-((5,6-diphenylpyrazin-yl)(isopropyl)amino)butyl)thio)-2,4-dihydro-3H-,12,4-triazol-3-one) is formed. (C) PK were assessed in mouse plasma over a 24-hour period. (D) Aggregation dose-response comparison of washed human platelets treated with vehicle, CS585 (3.125-100 nM), or 12-HETrE (500-15 000 nM), stimulated with collagen (0.5 μg/mL) (n = 4). (E) Integrin αIIbβ3 activation, α-granule, and dense granule secretion dose-response comparison of washed human platelets treated with vehicle, CS585 (3.125-100 nM) or 12-HETrE (500-15 000 nM) stimulated with convulxin (25 ng/mL) (n = 4). Data represent mean ± SEM. A one-way analysis of variance (ANOVA) was performed using an uncorrected Fisher least significant difference post hoc test. Asterisks denote statistical differences between the vehicle and treated groups: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. MFI, mean fluorescence intensity.
Potency of CS585 in human platelets
The potency of CS585 was initially assessed in washed human platelets using platelet aggregometry in response to thrombin, collagen, or adenosine 5′-diphosphate (ADP), all of which were endogenous agonists in the blood vessel. Platelets were treated with increasing concentrations of CS585 for 10 minutes (200 pM-100 nM) before stimulation with 0.5 nM thrombin, 0.5 μg/mL collagen, or 20 μM ADP. In all 3 conditions, platelet aggregation was inhibited by CS585 in a dose-dependent manner, with a 50% inhibitory concentration of 12.5 to 50 nM (Figure 2A-B). The assessment of the ability of CS585 to inhibit aggregation was repeated in PRP, because the albumin and immune cells present in PRP can often blunt or negate the ability of a compound to effectively impact platelet function. Aggregation was assessed after stimulation with either 1 or 2 μg/mL of collagen; CS585 was not observed to be dependent on collagen concentration to inhibit collagen-induced aggregation of PRP (Figure 2C-D). To determine whether the effects of CS585 could be overcome by higher agonist concentrations, inhibition of 1 μM CS585 on platelet aggregation was assessed after stimulation with increasing concentrations of agonists thrombin, collagen, or ADP. At 1 μM CS585, inhibtion could be overcome by 2.5 nM thrombin and partly by 10 μg/mL collagen, but not ADP (Figure 2E). Furthermore, washed platelets were treated with increasing concentrations of CS585 for 10 minutes (10 pM-100 nM) to assess the concentration at which CS585 began to activate PKA, which is an indicator of activation of a Gαs-coupled G-protein-coupled receptor (GPCR). After 10 minutes, the reaction was stopped and the level of phosphorylated VASP (Ser157 pVASP) was measured by western blot to determine the potency of CS585 in activating PKA. Surprisingly, VASP was observed to begin phosphorylation with as little as 10 pM CS585 and continued to show increasing levels of phosphorylation with increased CS585 concentrations that peaked at 25 nM (Figure 2F). To assess the potential of CS585 to inhibit integrin activation and granule secretion in the platelet, the activation of integrin αIIbβ3, α-, and dense granule secretion in response to 25 ng/mL convulxin was assessed by flow cytometry in washed human platelets. Convulxin was used as the agonist for this experiment because collagen requires a stirring or flowing condition for the active conformation of collagen to be exposed and allow binding to the glycoprotein VI receptor on the platelet. Using an antibody that recognizes only the active form of integrin αIIbβ3, CS585 was shown to fully inhibit integrin activity at 100 nM, with significant inhibition observed starting at 6.2 nM. Similarly, α-granule secretion was assessed by measuring the expression of P-selectin on the surface of the platelet, and dense granule secretion was assessed by measuring the expression of CD63 on the platelet surface after stimulation. For both of these markers, full inhibition of secretion was observed by 100 nM, whereas significant decreases in agonist-induced secretion were observed at concentrations of 6.2 nM for α-granule and 12.5 nM for dense granule secretion (Figure 2G; supplemental Figure 1). To determine whether the VASP phosphorylation observed in the presence of CS585 was mediated by adenylyl cyclase and not guanylyl cyclase, the intracellular levels of cAMP and cGMP were measured in washed human platelets after incubation with increasing concentrations of CS585 for 1 minute. A dose-dependent increase in cAMP levels was observed in the presence of CS585, but levels of cGMP remained unchanged (Figure 2H). VASP phosphorylation was also assessed in the presence of the PKA inhibitor H89. Pretreatment with H89 completely reversed the effects of CS585 on VASP phosphorylation in human platelets (Figure 2I-J).
CS585 potently inhibits human platelet activity. (A) Aggregation of washed human platelets treated with vehicle or CS585 (0.2-100 nM), stimulated with thrombin (0.5 nM), collagen (0.5 μg/mL), or ADP (20 μM). Data represent mean ± SEM. One-way ANOVA with Dunnett comparison (n = 4-6). (B) Representative aggregation curves for panel A. (C) Aggregation of human PRP treated with vehicle or CS585 (0.25-5 μM), stimulated with collagen (1 and 2 μg/mL, closed and open boxes, respectively) (n = 4). Data represent mean ± SEM. Mixed effects analysis with Dunnett comparison. Collagen aggregation of 1 μg/mL response compared with that of the vehicle; ∗∗P < .01; ∗∗∗∗P < .0001. Collagen aggregation of 2 μg/mL response compared with that of the vehicle; ##P < .01; ####P < .0001. (D) Representative aggregation curves for panel C. (E) Aggregation of washed human platelets treated with CS585 (1 μM), stimulated with thrombin (0.5-5 nM), collagen (0.5-10 μg/mL), or ADP (10-80 μM) (n = 4). (F) Expression of VASP phosphorylation (Ser157 VASP, 50 kilodalton [kDa]; total VASP, 46 and 50 kDa), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (37 kDa) on washed human platelets treated with vehicle or CS585 (10 pM-100 nM) for 10 minutes (n = 4). Representative blots are included above the graph. Data represent mean ± SEM. One-way ANOVA and Dunnett comparison. Asterisks denote statistical differences between vehicle and treated groups. (G) Activation of integrin αIIbβ3, α-granule, and dense granule secretion of washed human platelets treated vehicle or CS585 (6.2-100 nM), stimulated with convulxin (25 ng/mL) (n = 6). Data represent mean ± SEM. One-way ANOVA with Dunnett comparison. Asterisks denote statistical differences between vehicle and treated groups. (H) cAMP and cGMP levels measured in washed human platelets treated with vehicle, CS585 (0.1-100 nM), or positive controls forskolin (10 μM) and PAPA-NONOate (PAPA-NO, 5 μM) (n = 4). Data represent mean ± SEM. One-way ANOVA with Dunnett multiple comparison test. (I) Expression of Ser157 VASP (50 kDa) and GAPDH (37 kDa) in washed human platelets treated with vehicle or PKA inhibitor H89 (100 μM) before incubation with vehicle or CS585 (100 nM) (n = 6). Data represent mean ± SEM. One-way ANOVA with Tukey multiple comparison test. (J) Representative blot of panel I. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
CS585 potently inhibits human platelet activity. (A) Aggregation of washed human platelets treated with vehicle or CS585 (0.2-100 nM), stimulated with thrombin (0.5 nM), collagen (0.5 μg/mL), or ADP (20 μM). Data represent mean ± SEM. One-way ANOVA with Dunnett comparison (n = 4-6). (B) Representative aggregation curves for panel A. (C) Aggregation of human PRP treated with vehicle or CS585 (0.25-5 μM), stimulated with collagen (1 and 2 μg/mL, closed and open boxes, respectively) (n = 4). Data represent mean ± SEM. Mixed effects analysis with Dunnett comparison. Collagen aggregation of 1 μg/mL response compared with that of the vehicle; ∗∗P < .01; ∗∗∗∗P < .0001. Collagen aggregation of 2 μg/mL response compared with that of the vehicle; ##P < .01; ####P < .0001. (D) Representative aggregation curves for panel C. (E) Aggregation of washed human platelets treated with CS585 (1 μM), stimulated with thrombin (0.5-5 nM), collagen (0.5-10 μg/mL), or ADP (10-80 μM) (n = 4). (F) Expression of VASP phosphorylation (Ser157 VASP, 50 kilodalton [kDa]; total VASP, 46 and 50 kDa), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (37 kDa) on washed human platelets treated with vehicle or CS585 (10 pM-100 nM) for 10 minutes (n = 4). Representative blots are included above the graph. Data represent mean ± SEM. One-way ANOVA and Dunnett comparison. Asterisks denote statistical differences between vehicle and treated groups. (G) Activation of integrin αIIbβ3, α-granule, and dense granule secretion of washed human platelets treated vehicle or CS585 (6.2-100 nM), stimulated with convulxin (25 ng/mL) (n = 6). Data represent mean ± SEM. One-way ANOVA with Dunnett comparison. Asterisks denote statistical differences between vehicle and treated groups. (H) cAMP and cGMP levels measured in washed human platelets treated with vehicle, CS585 (0.1-100 nM), or positive controls forskolin (10 μM) and PAPA-NONOate (PAPA-NO, 5 μM) (n = 4). Data represent mean ± SEM. One-way ANOVA with Dunnett multiple comparison test. (I) Expression of Ser157 VASP (50 kDa) and GAPDH (37 kDa) in washed human platelets treated with vehicle or PKA inhibitor H89 (100 μM) before incubation with vehicle or CS585 (100 nM) (n = 6). Data represent mean ± SEM. One-way ANOVA with Tukey multiple comparison test. (J) Representative blot of panel I. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
CS585 demonstrates selectivity toward the IP receptor
One of the biggest challenges inherent to oxidized lipids, such as prostaglandins, is the apparent promiscuous nature of oxylipins to signal through multiple prostanoid receptors.23,24 This has created a significant challenge in the development of analogs that can selectively target the desired receptor without engaging similar receptors, which may result in unwanted effects.25 To determine which Gαs-coupled GPCR CS585 signals through, human platelets treated with CS585 were assessed in the absence or presence of receptor inhibitors for each Gαs-coupled GPCR expressed in the platelet. Each pharmacological inhibitor blocked the respective oxylipin from inhibiting platelet function (supplemental Figure 2). Although pretreatment with an inhibitor to block the DP1 receptor (MK-0524) or the EP2 and EP4 receptors (TG4-155 and CJ-42794, respectively) had no effect on CS585 inhibition of platelet aggregation, inhibition of the IP receptor (Ro 1138542) completely blocked CS585-mediated inhibition of collagen-induced platelet aggregation (Figure 3A-B). Similarly, CS585 selectively attenuated integrin αIIbβ3 activation, α-granule secretion, and dense granule secretion in human platelets through the IP receptor but not through the DP1, EP2, or EP4 receptors (Figure 3C; supplemental Figure 3). In fact, Ro 1138452 completely reversed the effects of CS585 on markers of platelet activation (supplemental Figure 4). Furthermore, CS585-induced phosphorylation of VASP was blocked only through the inhibition of the IP receptor but not of the DP1, EP2, or EP4 receptors (Figure 3D). To support the IP receptor as being required for CS585 phosphorylation of VASP, washed platelets from WT and IP−/− mice were assessed for VASP phosphorylation after treatment with 12-HETrE or CS585. Platelets from WT mice showed VASP phosphorylation after treatment with either 12-HETrE or CS585; however, platelets from IP−/− mice showed a lack of phosphorylated VASP in the presence of either 12-HETrE or CS585 (Figure 3E; supplemental Figure 5). Forskolin, a compound that directly activates adenylyl cyclase downstream of the receptor, was unaffected by its ability to phosphorylate VASP in platelets of both WT and IP−/− mice.
CS585 is selective to activation of the IP receptor. (A) Aggregation of washed human platelets treated with vehicle or an IP receptor inhibitor (Ro 1138542, 5 μM), DP1 receptor inhibitor (MK-0542, 4 nM), or EP2 and EP4 receptor inhibitors (TG4-155, 2 μM and CJ-42794, 80 nM, respectively) before treatment with vehicle or CS585 (0.05-100 nM) stimulated with collagen (0.5 μg/mL; n = 4-5). Data represent mean ± SEM. Two-factor mixed-effects analysis with Tukey multiple comparisons test. Each condition is compared with its corresponding vehicle. (B) Representative aggregation curves for panel A. (C) Activation of integrin αIIbβ3, α-granule, and dense granule secretion of washed human platelets treated with the vehicle or an IP receptor inhibitor (Ro 1138542, 5 μM), DP1 receptor inhibitor (MK-0542, 4 nM), or EP2 and EP4 receptor inhibitors (TG4-155, 2 μM and CJ-42794, 80 nM) before treatment with the vehicle or CS585 (12.5-100 nM), stimulated with convulxin (25 ng/mL) (n = 4-5). The results are expressed as the relative mean (percentage of vehicle MFI) ± SEM. Two-factor mixed-effects analysis with Sidak multiple comparisons. Each condition is compared with the corresponding vehicle. (D) Expression of Ser157 pVASP (50 kDa) and GAPDH (37 kDa) in washed human platelets treated with vehicle or an IP receptor inhibitor (Ro 1138542, 5 μM), DP1 receptor inhibitor (MK-0542, 4 nM), or EP2 and EP4 receptor inhibitors (TG4-155, 2 μM and CJ-42794, 80 nM, respectively) before treatment with vehicle or CS585 (10 pM-100 nM; n = 4). Data represent mean ± SEM. Two-way ANOVA with Dunnett correction. Asterisks denote statistical differences between vehicle and treated groups. (E) Expression of Ser157 pVASP (50 kDa) and GAPDH (37 kDa) in washed platelets from WT and IP receptor-deficient (IP−/−) mice treated with vehicle, CS585 (1, 5, or 10 μM), 12-HETrE (10 or 25 μM), or forskolin (5 μM; n = 3; 2 mice pooled per n). ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001.
CS585 is selective to activation of the IP receptor. (A) Aggregation of washed human platelets treated with vehicle or an IP receptor inhibitor (Ro 1138542, 5 μM), DP1 receptor inhibitor (MK-0542, 4 nM), or EP2 and EP4 receptor inhibitors (TG4-155, 2 μM and CJ-42794, 80 nM, respectively) before treatment with vehicle or CS585 (0.05-100 nM) stimulated with collagen (0.5 μg/mL; n = 4-5). Data represent mean ± SEM. Two-factor mixed-effects analysis with Tukey multiple comparisons test. Each condition is compared with its corresponding vehicle. (B) Representative aggregation curves for panel A. (C) Activation of integrin αIIbβ3, α-granule, and dense granule secretion of washed human platelets treated with the vehicle or an IP receptor inhibitor (Ro 1138542, 5 μM), DP1 receptor inhibitor (MK-0542, 4 nM), or EP2 and EP4 receptor inhibitors (TG4-155, 2 μM and CJ-42794, 80 nM) before treatment with the vehicle or CS585 (12.5-100 nM), stimulated with convulxin (25 ng/mL) (n = 4-5). The results are expressed as the relative mean (percentage of vehicle MFI) ± SEM. Two-factor mixed-effects analysis with Sidak multiple comparisons. Each condition is compared with the corresponding vehicle. (D) Expression of Ser157 pVASP (50 kDa) and GAPDH (37 kDa) in washed human platelets treated with vehicle or an IP receptor inhibitor (Ro 1138542, 5 μM), DP1 receptor inhibitor (MK-0542, 4 nM), or EP2 and EP4 receptor inhibitors (TG4-155, 2 μM and CJ-42794, 80 nM, respectively) before treatment with vehicle or CS585 (10 pM-100 nM; n = 4). Data represent mean ± SEM. Two-way ANOVA with Dunnett correction. Asterisks denote statistical differences between vehicle and treated groups. (E) Expression of Ser157 pVASP (50 kDa) and GAPDH (37 kDa) in washed platelets from WT and IP receptor-deficient (IP−/−) mice treated with vehicle, CS585 (1, 5, or 10 μM), 12-HETrE (10 or 25 μM), or forskolin (5 μM; n = 3; 2 mice pooled per n). ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001.
CS585 attenuates ex vivo platelet adhesion and thrombus formation under flow
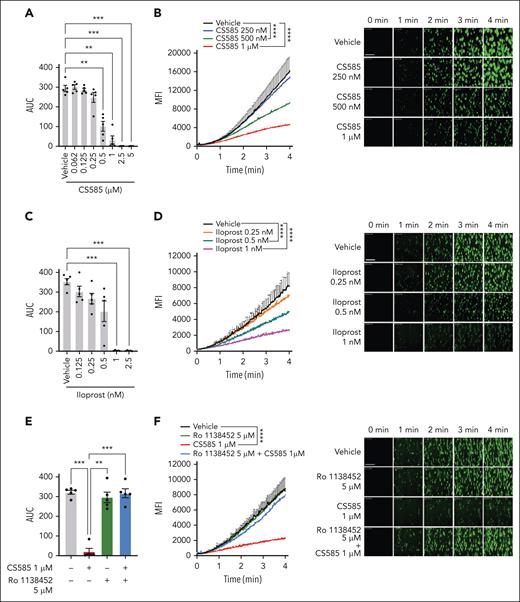
To determine whether the inhibitory effects of CS585 observed in vitro could be recapitulated in a more physiological setting, platelet adhesion and thrombus formation were assessed ex vivo using the microfluidic perfusion flow chamber and total thrombus formation analysis system (T-TAS) assays, respectively. Increases in the pressure of human whole blood perfused over a collagen-coated PL chip were monitored in the presence of increasing concentrations of CS585 in T-TAS. In a parallel set of experiments using a microfluidic perfusion flow system, whole blood treated with DiOC6 to stain platelets was perfused over a channel coated with collagen under arterial flow conditions, and platelet adhesion and accumulation at the collagen-coated surface were measured. These experiments were conducted using both CS585 and iloprost, which is a well-characterized IP receptor agonist. Increasing concentrations of CS585 demonstrated a decrease in the AUC; 500 nM CS585 significantly decreased the AUC, whereas 1 μM CS585 completely prevented clot formation (Figure 4A; supplemental Figure 6A). Additionally, CS585 was shown to reduce platelet adherence to the collagen-coated surface; the rate of platelet adhesion to collagen at high shear was significantly decreased at 500 nM CS585 (Figure 4B). Whole blood treated with iloprost and assessed using T-TAS demonstrated a significant decrease in the AUC (Figure 4C; supplemental Figure 6B). In the perfusion flow chamber, iloprost was also observed to significantly decrease platelet adhesion (Figure 4D). To determine whether the IP receptor inhibitor (Ro 1138452) could reverse the effects of CS585, whole blood pretreated with Ro 1138452 was incubated with CS585. In both ex vivo perfusion assays, pretreatment with Ro 1138452 completely recovered the physiologic response in the presence of CS585 (Figure 4E-F; supplemental Figure 6C).
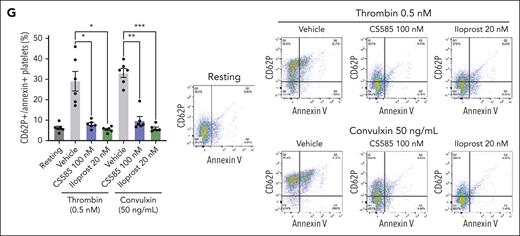
CS585 attenuates ex vivo platelet adhesion and thrombus formation under flow. (A) Human whole blood treated with vehicle or CS585 (0.062-5 μM) was perfused on a collagen-coated PL chip at arterial shear using T-TAS (n = 5). Data represent mean ± SEM. One-way ANOVA with Dunnett correction. (B) Quantification and representative images of platelet adhesion and accumulation in human whole blood stained with DiOC6 and treated with vehicle or CS585 (250 nM-1 μM) perfused through a collagen-coated chamber at arterial shear (n = 6). Data represent mean ± SEM. Two-way ANOVA. Scale bars represent 100 μm. (C) Human whole blood treated with vehicle or iloprost (0.125-2.5 nM) and then perfused on a collagen-coated PL chip at arterial shear using T-TAS (n = 5). Data represent mean ± SEM. One-way ANOVA with Dunnett correction. (D) Quantification and representative images of platelet adhesion and accumulation of human whole blood stained with DiOC6 and treated with vehicle or iloprost (0.25-1 nM) perfused through a collagen-coated chamber at arterial shear (n = 6). Data represent mean ± SEM. Two-way ANOVA. Scale bars represent 100 μm. (E) Human whole blood treated with vehicle or an IP receptor inhibitor (Ro 1138542, 5 μM) before treatment with vehicle or CS585 (1 μM) perfused on a collagen-coated PL chip at arterial shear using T-TAS (n = 5). Data represent mean ± SEM. One-way ANOVA with Tukey multiple comparison test. (F) Quantification and representative images of platelet adhesion and accumulation in human whole blood stained with DiOC6 and treated with vehicle or an IP receptor inhibitor (Ro 1138542, 5 μM) before treatment with vehicle or CS585 (1 μM) perfused through a collagen-coated chamber under arterial shear (n = 6). Data represent mean ± SEM. Two-way ANOVA. Scale bars represent 100 μm. (G) Quantification and representative contour plots of phosphatidylserine exposure in washed human platelets treated with vehicle, CS585 (100 nM), or iloprost (20 nM), incubated with annexin V and an antibody for CD62P, then stimulated with 0.5 nM thrombin or 50 ng/mL (n = 5). Data represent mean percentage of dual-positive platelets ± SEM. One-way ANOVA with Tukey multiple comparison test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
CS585 attenuates ex vivo platelet adhesion and thrombus formation under flow. (A) Human whole blood treated with vehicle or CS585 (0.062-5 μM) was perfused on a collagen-coated PL chip at arterial shear using T-TAS (n = 5). Data represent mean ± SEM. One-way ANOVA with Dunnett correction. (B) Quantification and representative images of platelet adhesion and accumulation in human whole blood stained with DiOC6 and treated with vehicle or CS585 (250 nM-1 μM) perfused through a collagen-coated chamber at arterial shear (n = 6). Data represent mean ± SEM. Two-way ANOVA. Scale bars represent 100 μm. (C) Human whole blood treated with vehicle or iloprost (0.125-2.5 nM) and then perfused on a collagen-coated PL chip at arterial shear using T-TAS (n = 5). Data represent mean ± SEM. One-way ANOVA with Dunnett correction. (D) Quantification and representative images of platelet adhesion and accumulation of human whole blood stained with DiOC6 and treated with vehicle or iloprost (0.25-1 nM) perfused through a collagen-coated chamber at arterial shear (n = 6). Data represent mean ± SEM. Two-way ANOVA. Scale bars represent 100 μm. (E) Human whole blood treated with vehicle or an IP receptor inhibitor (Ro 1138542, 5 μM) before treatment with vehicle or CS585 (1 μM) perfused on a collagen-coated PL chip at arterial shear using T-TAS (n = 5). Data represent mean ± SEM. One-way ANOVA with Tukey multiple comparison test. (F) Quantification and representative images of platelet adhesion and accumulation in human whole blood stained with DiOC6 and treated with vehicle or an IP receptor inhibitor (Ro 1138542, 5 μM) before treatment with vehicle or CS585 (1 μM) perfused through a collagen-coated chamber under arterial shear (n = 6). Data represent mean ± SEM. Two-way ANOVA. Scale bars represent 100 μm. (G) Quantification and representative contour plots of phosphatidylserine exposure in washed human platelets treated with vehicle, CS585 (100 nM), or iloprost (20 nM), incubated with annexin V and an antibody for CD62P, then stimulated with 0.5 nM thrombin or 50 ng/mL (n = 5). Data represent mean percentage of dual-positive platelets ± SEM. One-way ANOVA with Tukey multiple comparison test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
CS585 reduces PS exposure after stimulation in human platelets
Iloprost decreases surface phosphatidylserine (PS) exposure in platelets.26 To determine whether CS585 had a similar effect, washed human platelets were incubated with CS585 or iloprost before stimulation with either thrombin or convulxin. Platelets were dual stained for PS (annexin V) and P-selectin (CD62P), and the percentage of the platelet population positive for both PS and P-selectin was compared. Both CS585 and iloprost significantly reduced the percentage of dual-positive events in platelets stimulated with either convulxin or thrombin (Figure 4G).
In vivo stability of CS585 and its efficacy in attenuating thrombosis
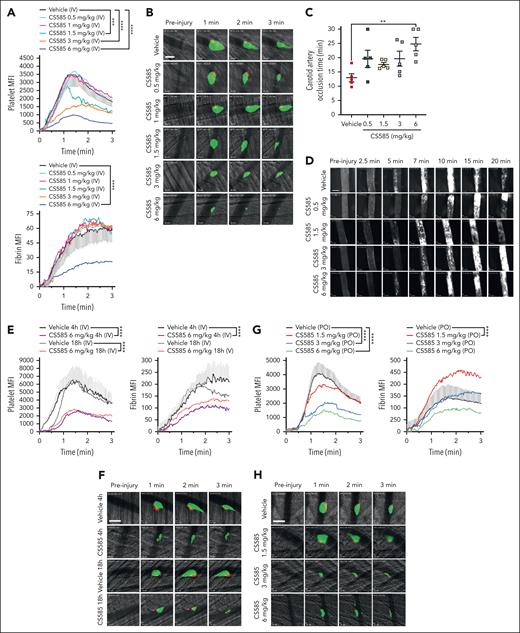
To determine whether the effects of CS585 observed in vitro and ex vivo translate to the regulation of clot formation and occlusive thrombus formation in vivo, mice were treated either IV or by mouth with CS585 and the degree of thrombus formation after vascular injury was assessed using real-time intravital microscopy. To assess whether platelet accumulation and fibrin formation at the site of vascular injury is attenuated by CS585, increasing doses of CS585 (0.5-6 mg/kg) were administered IV to the mouse 10 minutes before induction of a laser-induced injury in the cremaster arteriole. CS585-treated mice demonstrated a significant decrease in platelet clot formation at the site of injury at doses of 1.5 to 6 mg/kg (Figure 5A-B) compared with vehicle-treated mice. Interestingly, fibrin formation was unaltered in all except the 6 mg/kg condition. Subsequently, occlusive thrombus formation was assessed in the presence of CS585 in the carotid artery after application of 10% FeCl3 to the carotid artery for 2 minutes to denude the endothelium. Increasing concentrations of CS585 were administered IV 15 minutes before FeCl3, followed by microscopic measurements of platelet accumulation at the site of injury to determine the occlusion time of the vessel. The occlusion time in vehicle-treated animals was 12.9 ± 1.4 minutes, while treatment with 6 mg/kg of CS585 significantly delayed occlusion time in the carotid artery of these animals (24.8 ± 2.3 minutes; Figure 5C-D).
CS585 prevents thrombosis in small and large vessels. (A) WT mice were administered vehicle or CS585 (0.5-6 mg/kg), followed by the induction of cremaster arteriole laser-induced injury to assess platelet and fibrin accumulation at the site of injury (n = 3 mice per condition/8 injuries per mouse). Data represent mean ± SEM. Two-way ANOVA. (B) Representative images of panel A showing platelet plug formation (green) and fibrin accumulation (red). Scale bars represent 50 μm. (C) WT mice were administered vehicle or CS585 (0.5-6 mg/kg) and the carotid artery FeCl3-induced thrombosis model was assessed (n = 5). Data represent mean ± SEM. One-way ANOVA with Dunnett correction. (D) Representative images of panel C. Scale bars represent 500 μm. (E) WT mice were administered vehicle or CS585 (6 mg/kg) and the stability of CS585 in the blood was determined by induction of the cremaster arteriole laser-induced injury to assess platelet and fibrin accumulation at the site of injury at 4 hours and 18 hours after administration (n = 3 mice per condition/8 injuries per mouse). Data represent mean ± SEM. Two-way ANOVA. (F) Representative images of panel E, showing platelet plug formation (green) and fibrin accumulation (red). Scale bars represent 50 μm. (G) WT mice were administered a single dose of vehicle or CS585 (1.5-6 mg/kg) per oral (PO) and the functional efficacy of CS585 in the blood was determined by cremaster arteriole laser-induced injury to assess platelet and fibrin accumulation at the site of injury 4 hours after administration (n = 3 mice per condition/8 injuries per mouse). Data represent mean ± SEM. Two-way ANOVA. (H) Representative images of panel G, showing platelet plug formation (green) and fibrin accumulation (red). Scale bars represent 50 μm. (I) WT mice were administered vehicle or CS585 (3 mg/kg) PO and the stability of CS585 in the blood was determined by cremaster arteriole laser-induced injury to assess platelet and fibrin accumulation at the site of injury 24, 48, and 72 hours after administration (n = 3 mice per condition/8 injuries per mouse). Data represent mean ± SEM. Two-way ANOVA. (J) Representative images of panel I, showing platelet plug formation (green) and fibrin accumulation (red). Scale bars represent 50 μm. (K) Quantification of phosphatidylserine exposure in washed platelets from WT mice IV administered with vehicle or CS585 (6 mg/kg; CS585 [IV]) and stained with annexin V, stimulated with convulxin (50 ng/mL). Washed platelets from untreated WT mice treated ex vivo with CS585 (10 μM) before annexin V staining and stimulation with convulxin (50 ng/mL) (n = 5). Quantification of the percentage of annexin V–positive events represent the levels of PS exposure. Data represent mean ± SEM. One-way ANOVA with Dunnett multiple comparisons. (L) WT mice treated with vehicle or CS585 (6 mg/kg) daily starting 24 hours before thrombus initiation. The venous thrombus mass was measured 2 days after inferior vena cava ligation (n = 5). Data represent mean ± SEM. Welch t-test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
CS585 prevents thrombosis in small and large vessels. (A) WT mice were administered vehicle or CS585 (0.5-6 mg/kg), followed by the induction of cremaster arteriole laser-induced injury to assess platelet and fibrin accumulation at the site of injury (n = 3 mice per condition/8 injuries per mouse). Data represent mean ± SEM. Two-way ANOVA. (B) Representative images of panel A showing platelet plug formation (green) and fibrin accumulation (red). Scale bars represent 50 μm. (C) WT mice were administered vehicle or CS585 (0.5-6 mg/kg) and the carotid artery FeCl3-induced thrombosis model was assessed (n = 5). Data represent mean ± SEM. One-way ANOVA with Dunnett correction. (D) Representative images of panel C. Scale bars represent 500 μm. (E) WT mice were administered vehicle or CS585 (6 mg/kg) and the stability of CS585 in the blood was determined by induction of the cremaster arteriole laser-induced injury to assess platelet and fibrin accumulation at the site of injury at 4 hours and 18 hours after administration (n = 3 mice per condition/8 injuries per mouse). Data represent mean ± SEM. Two-way ANOVA. (F) Representative images of panel E, showing platelet plug formation (green) and fibrin accumulation (red). Scale bars represent 50 μm. (G) WT mice were administered a single dose of vehicle or CS585 (1.5-6 mg/kg) per oral (PO) and the functional efficacy of CS585 in the blood was determined by cremaster arteriole laser-induced injury to assess platelet and fibrin accumulation at the site of injury 4 hours after administration (n = 3 mice per condition/8 injuries per mouse). Data represent mean ± SEM. Two-way ANOVA. (H) Representative images of panel G, showing platelet plug formation (green) and fibrin accumulation (red). Scale bars represent 50 μm. (I) WT mice were administered vehicle or CS585 (3 mg/kg) PO and the stability of CS585 in the blood was determined by cremaster arteriole laser-induced injury to assess platelet and fibrin accumulation at the site of injury 24, 48, and 72 hours after administration (n = 3 mice per condition/8 injuries per mouse). Data represent mean ± SEM. Two-way ANOVA. (J) Representative images of panel I, showing platelet plug formation (green) and fibrin accumulation (red). Scale bars represent 50 μm. (K) Quantification of phosphatidylserine exposure in washed platelets from WT mice IV administered with vehicle or CS585 (6 mg/kg; CS585 [IV]) and stained with annexin V, stimulated with convulxin (50 ng/mL). Washed platelets from untreated WT mice treated ex vivo with CS585 (10 μM) before annexin V staining and stimulation with convulxin (50 ng/mL) (n = 5). Quantification of the percentage of annexin V–positive events represent the levels of PS exposure. Data represent mean ± SEM. One-way ANOVA with Dunnett multiple comparisons. (L) WT mice treated with vehicle or CS585 (6 mg/kg) daily starting 24 hours before thrombus initiation. The venous thrombus mass was measured 2 days after inferior vena cava ligation (n = 5). Data represent mean ± SEM. Welch t-test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
To determine the duration of the effects of CS585, 6 mg/kg CS585 was administered IV, and laser-induced injury of the cremaster arteriole was conducted at either 4 hours or 18 hours after administration. In both conditions, single IV treatment with CS585 was able to significantly delay and reduce the degree of platelet accumulation as well as the amount of fibrin (4 hours after administration) at the site of injury (Figure 5E-F). To determine whether CS585 crosses the gut wall and is orally available, increasing doses of CS585 (1.5-6 mg/kg) were administered by mouth, and laser-induced injury in the cremaster arteriole was performed. CS585 doses of 3 and 6 mg/kg were able to significantly attenuate platelet plug formation at the site of injury 4 hours after administration (Figure 5G-H). To assess in vivo stability after oral administration, mice were dosed with 3 mg/kg CS585, and laser-induced injury of the cremaster arteriole was performed 24, 48, and 72 hours after administration. CS585 prevented platelet accumulation at the 24- and 48-hour time points, but the response returned to the level of the vehicle at 72 hours after administration (Figure 5I-J). These effects were corroborated by VASP phosphorylation data, in which continued VASP phosphorylation in human platelets was observed for up to 24 hours after ex vivo incubation with 100 nM CS585 (supplemental Figure 7).
CS585 reduces procoagulant activity in mouse platelets
The effects of 6 mg/kg CS585 on platelet procoagulant activity were assessed. Four hours after IV treatment with either vehicle or CS585, washed platelets were stimulated with convulxin, and annexin V binding was assessed. Washed platelets from mice administered CS585 as well as platelets from vehicle-dosed mice treated ex vivo with CS585 displayed significantly lower levels of annexin V–positive events than the vehicle (Figure 5K).
CS585 impairs experimental venous thrombosis
To assess the effects of CS585 in an in vivo model of venous thrombosis, venous thrombosis was induced in WT mice by stasis of the inferior vena cava. After induction, mice were dosed with 6 mg/kg CS585 every 24 hours for 2 days. On day 2 after thrombosis induction, the mice were euthanized, and the thrombus was resected and measured to determine the thrombus mass (g/cm). Consistent with data demonstrating that platelet depletion impairs experimental venous thrombosis,27,28 platelet inhibition with CS585 treatment after thrombus induction resulted in a significant reduction in the acute venous thrombus mass (Figure 5L).
CS585 regulates platelet function independently of coagulation and does not increase the risk of bleeding
Targeting platelet reactivity is the primary mechanism that decreases the risk of thrombosis. However, decreased platelet reactivity may result in an increased risk of bleeding, which can result in significant morbidity and mortality.1,29 To determine whether the decreased thrombotic risk resulting from CS585 treatment shown in Figure 5 alters coagulation or bleeding risk, mice were treated IV with vehicle or 2 doses of CS585 (0.5 or 6 mg/kg). After administration of CS585, the distal 5 mm of the tail was resected, and the tail was placed in saline at 37°C. The elapsed time between the resection of the tail and cessation of bleeding was recorded as the tail bleeding time. Neither treatment with 0.5 mg/kg nor 6 mg/kg CS585 resulted in a significant increase in the tail bleeding time in mice (Figure 6A). To further assess the potential for altering coagulation in the blood after CS585 administration, coagulation was assessed in human whole blood treated with CS585 using TEG. TEG activates the contact pathway in the blood, and by following TEG curves, it is possible to assess the changes in coagulation activity and clotting time. Six conditions were tested using TEG in this study: vehicle, 2 doses of CS585, rivaroxaban, and 2 doses of CS585 + rivaroxaban. Neither the TEG parameters nor thrombin formation were altered in the blood treated with CS585 compared with the vehicle condition (Figure 6B-H). Rivaroxaban, a Food and Drug Administration–approved factor Xa inhibitor, showed a significant delay in the initiation time for clotting. Finally, the addition of CS585 to rivaroxaban did not significantly shift the TEG curve compared with rivaroxaban alone, suggesting that CS585 does not negatively interact with the coagulation system.
CS585 does not alter hemostasis. (A) WT mice were treated with vehicle or CS585 (0.5 or 6 mg/kg), and bleeding time of the tail was assessed (n = 10). Data represent mean ± SEM. One-way ANOVA with Dunnett correction. (B-E) Human whole blood was treated with the vehicle, CS585 (100 nM or 1 μM), rivaroxaban (250 ng/mL), or CS585 (100 nM or 1 μM) + rivaroxaban (250 ng/mL). Coagulation parameters were assessed using TEG (n = 4). (B) Reaction time is the time to initial fibrin thread formation (minutes). (C) Maximum amplitude and the maximum strength of the clot (mm). (D) K time represents the time until the clot reaches a strength of 20 mm (minutes). (E) α angle, rate of clot formation (degree). One-way ANOVA with Dunnett correction. (F) Representative tracing of the coagulation parameters measured in panels B-E. (G) The maximum rate of thrombin generation was measured by TEG using a velocity curve that graphs the clot strength over time (n = 4). One-way ANOVA with Dunnett correction. (H) Representative tracing of the velocity curve used to calculate the rate of thrombin generation. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, nonsignificant.
CS585 does not alter hemostasis. (A) WT mice were treated with vehicle or CS585 (0.5 or 6 mg/kg), and bleeding time of the tail was assessed (n = 10). Data represent mean ± SEM. One-way ANOVA with Dunnett correction. (B-E) Human whole blood was treated with the vehicle, CS585 (100 nM or 1 μM), rivaroxaban (250 ng/mL), or CS585 (100 nM or 1 μM) + rivaroxaban (250 ng/mL). Coagulation parameters were assessed using TEG (n = 4). (B) Reaction time is the time to initial fibrin thread formation (minutes). (C) Maximum amplitude and the maximum strength of the clot (mm). (D) K time represents the time until the clot reaches a strength of 20 mm (minutes). (E) α angle, rate of clot formation (degree). One-way ANOVA with Dunnett correction. (F) Representative tracing of the coagulation parameters measured in panels B-E. (G) The maximum rate of thrombin generation was measured by TEG using a velocity curve that graphs the clot strength over time (n = 4). One-way ANOVA with Dunnett correction. (H) Representative tracing of the velocity curve used to calculate the rate of thrombin generation. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, nonsignificant.
Discussion
The discovery of oxylipin 12-HETrE and delineation of its function in regulating platelet reactivity through the IP receptor provides an opportunity to revisit the IP receptor as a potential primary target for the regulation of hemostasis and thrombosis.6,7,22 Using 12-HETrE as a guide, we were able to develop an analog with several unique attributes that may represent a renewed opportunity for effectively targeting thrombosis by targeting and activating the IP receptor.30,31 Here, CS585 is shown to function as a potent IP receptor agonist. In contrast to other IP receptor agonists such as iloprost and selexipag, CS585 was shown to maintain a sustained effect in the blood. Additionally, although current targets for the IP receptor are published to act promiscuously, binding to multiple prostaglandin receptors in addition to the IP receptor,25,32,33 we demonstrated that CS585 shows selectivity toward the IP receptor. Hence, CS585 represents a novel approach for targeting a receptor with the potential for effective inhibition of platelet reactivity and subsequent clot formation.
Although current antiplatelet targets have successfully decreased morbidity and mortality in cardiovascular disease, complications due to thrombosis remain a significant health concern.34 Furthermore, all of these targeted interventions have resulted in an increased risk of bleeding or off-target effects that limit their tolerance and utility in treatment.1,2,29,35 The IP receptor has been investigated in the past, but its implementation and acceptance by the Food and Drug Administration have been limited by its lack of stability in the blood and off-target effects.30 In contrast to the initial development path taken by other IP receptor agonists using prostaglandin PGI2, which has an inherently short half-life, as a guide for development,25,30 we used the 12-lipoxygenase oxylipin, 12-HETrE, as the origin for the development of CS585. The synthetic design used for CS585 development resulted in a highly stable compound that retained the beneficial characteristics of an IP receptor agonist without the challenges of selectivity and stability (Figure 1A-B). CS585 was shown to attenuate thrombin, collagen, and ADP-mediated platelet activation (Figure 2A-E,G), supporting its potential utility in decreasing platelet reactivity after exposure to multiple endogenous platelet agonists. Here, we showed that the inhibitory effects of CS585 were the result of activation of the IP receptor, leading to increased cAMP production and subsequent activation of PKA, which is known to play a key role in the inhibition of platelet activity (Figure 2F-J). The effects of CS585 observed in vitro were maintained in whole blood ex vivo settings, mimicking the previously observed properties of 12-HETrE.8
Sustained effects in the blood are a challenge that has excluded a number of drug targets from being translated from the bench to clinical practice. To determine whether CS585 has the physical and functional characteristics necessary for a sustained effect in the blood, PK and pharmacodynamics in several conditions were assessed, including a 21-day stability test, PK in mice by mouth administration (Figure 1C), IV administration in mice for up to 18 hours, and oral administration in mice for at least 48 hours (Figure 5A-J). CS585 demonstrated robust stability in both its PK and pharmacodynamic properties. Hence, the stability profile for CS585 suggests that it has the potential, based on the studies presented here, to be administered as either an IV or oral compound and to have long-term (multiday) protective effects in the blood. Importantly, the orally administered effects were shown to be reversible in vivo.
CS585 was observed to protect against clot formation in the vessels in several different in vivo thrombosis models in mice (Figure 5A-J). Although procoagulant studies have demonstrated a link between CS585 treatment and the ability of the platelet to express PS on its surface, the extent to which this effect is physiologically relevant remains unknown due to the involvement of endothelial PS.36 Furthermore, TEG studies support the lack of effect of CS585 on coagulation in the blood. Bleeding is a significant concern and a risk that has been observed with many antiplatelet agents currently in use.35 To identify whether CS585 exhibits a bleeding risk, mice treated with CS585 underwent a tail vein bleeding assay in which the distal 5 mm of the tail was resected and the time to bleeding cessation was measured. CS585 was not found to exhibit an increase in bleeding time, even at the highest dose of 6 mg/kg, suggesting that bleeding may not be a risk factor with inhibition from CS585 (Figure 6).
CS585 was developed as a novel approach to regulate platelet function through the selective activation of the IP receptor. CS585 exhibits a sustained effect in blood and plasma, can be administered IV or orally, and is highly effective in limiting platelet activation, clotting, and thrombosis. Although these data are promising, it is important to note that in addition to the platelet, vascular smooth muscle cells also express the IP receptor at high levels.37,38 Interestingly, activation of the IP receptor in vascular smooth muscle cells has been shown to have cardioprotective effects, highlighting a potential additional indication for CS585.39,40
Although much work remains to translate this novel compound into an effective new drug for the prevention of a number of pathophysiological conditions leading to thrombosis, the work presented here is a major advancement in this field. We have shown that derivatives of oxylipin lipids8,22 may represent a novel class of drugs for the prevention of thrombosis. Hence, CS585 has long-term potential to be used in the clinical treatment of cardiovascular diseases triggered by thrombosis, such as arterial thrombosis and venous thrombus embolism.
Acknowledgments
The authors thank Amanda Prieur (University of Michigan) for recruiting and consenting donors for the human studies. The authors thank Garrett FitzGerald (University of Pennsylvania) for providing prostacyclin receptor–deficient (IP−/−) mice.
This work was supported by the National Institutes of Health (NIH), National Institute of General Medical Sciences (NIGMS) grant GM131835 (M.H.), NIH, National Heart, Lung, and Blood Institute (NHLBI) grant HL007853 (A.Y.), NIH, NIGMS grant GM140223 (L.S.), NIH, NHLBI grant 5K08HL155408 (A.T.O.), NIH, National Center for Advancing Translational Sciences grant TR002240 (M.H., A.Y.), American Heart Association grant 23PRE1019986 (L.S.), and the Baiardi Family Foundation (A.T.O.). Cereno Scientific provided research funds for this study.
Authorship
Contribution: M.H., L.S., A.Y., B.D., N.B., A.W., and A.T.O. designed the study; L.S., A.Y., P.Y., S.L., A.R., D.G., C.L., K.K., and M.H. conducted and evaluated the experiments; and M.H., L.S., A.Y., S.L., B.D., A.W., N.B., and A.T.O. wrote and edited the manuscript.
Conflict-of-interest disclosure: M.H. is an equity holder and serves on the scientific advisory board for Veralox Therapeutics; is an equity holder and consultant for Cereno Scientific; and has received research funding from Cereno Scientific for this project. Additionally, M.H. and A.W. are listed as inventors of CS585 with associated patents US11,236,044 and US11,498,905. B.D. is the Chief Medical Officer for Cereno Scientific and is an equity holder. N.B. serves as a scientific adviser for Cereno Scientific and is an equity holder. The remaining authors declare no competing financial interests.
Correspondence: Michael Holinstat, Department of Pharmacology, University of Michigan, 1150 West Medical Center Dr, 2220D Medical Sciences Research Building 3, Ann Arbor, MI 48109-5632; e-mail: mholinst@umich.edu.
References
Author notes
∗L.S. and A.Y. contributed equally to this work.
Data are available on request from the corresponding author, Michael Holinstat (mholinst@umich.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



![CS585 potently inhibits human platelet activity. (A) Aggregation of washed human platelets treated with vehicle or CS585 (0.2-100 nM), stimulated with thrombin (0.5 nM), collagen (0.5 μg/mL), or ADP (20 μM). Data represent mean ± SEM. One-way ANOVA with Dunnett comparison (n = 4-6). (B) Representative aggregation curves for panel A. (C) Aggregation of human PRP treated with vehicle or CS585 (0.25-5 μM), stimulated with collagen (1 and 2 μg/mL, closed and open boxes, respectively) (n = 4). Data represent mean ± SEM. Mixed effects analysis with Dunnett comparison. Collagen aggregation of 1 μg/mL response compared with that of the vehicle; ∗∗P < .01; ∗∗∗∗P < .0001. Collagen aggregation of 2 μg/mL response compared with that of the vehicle; ##P < .01; ####P < .0001. (D) Representative aggregation curves for panel C. (E) Aggregation of washed human platelets treated with CS585 (1 μM), stimulated with thrombin (0.5-5 nM), collagen (0.5-10 μg/mL), or ADP (10-80 μM) (n = 4). (F) Expression of VASP phosphorylation (Ser157 VASP, 50 kilodalton [kDa]; total VASP, 46 and 50 kDa), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (37 kDa) on washed human platelets treated with vehicle or CS585 (10 pM-100 nM) for 10 minutes (n = 4). Representative blots are included above the graph. Data represent mean ± SEM. One-way ANOVA and Dunnett comparison. Asterisks denote statistical differences between vehicle and treated groups. (G) Activation of integrin αIIbβ3, α-granule, and dense granule secretion of washed human platelets treated vehicle or CS585 (6.2-100 nM), stimulated with convulxin (25 ng/mL) (n = 6). Data represent mean ± SEM. One-way ANOVA with Dunnett comparison. Asterisks denote statistical differences between vehicle and treated groups. (H) cAMP and cGMP levels measured in washed human platelets treated with vehicle, CS585 (0.1-100 nM), or positive controls forskolin (10 μM) and PAPA-NONOate (PAPA-NO, 5 μM) (n = 4). Data represent mean ± SEM. One-way ANOVA with Dunnett multiple comparison test. (I) Expression of Ser157 VASP (50 kDa) and GAPDH (37 kDa) in washed human platelets treated with vehicle or PKA inhibitor H89 (100 μM) before incubation with vehicle or CS585 (100 nM) (n = 6). Data represent mean ± SEM. One-way ANOVA with Tukey multiple comparison test. (J) Representative blot of panel I. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/18/10.1182_blood.2023020622/2/m_blood_bld-2023-020622-gr2.jpeg?Expires=1765884025&Signature=pVnuCiA8JbMo42U~IAfboT2jjUOKIy~DeRsDbCCxUACN7eTEqLcMKCoImVbhlP5-llwtsXMD4eLOuHR1KxhboTkkN7QwFOr9q0GQIzOtacG7Md4-PhZcHpzp~t6jPqRgZSurxHt9OfN5Yhskw8lge4zfoSIUuc~DJtRzj1sz37yQR1EJzhQ1SBYMfjHgG4f6M5bAXTMATAFmilWBJSpyuhEasyS96ruma3ulA85elt3TYx7VMBcasaQIhlupZQ95W57Y4k30cr-o2JHCxdYV0IFxXnmpsGdG9xLJREQoDMBJlVo6~7NCl8rgJ4IlC-yNEwjkmIK-oSnHoKizeEnLMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![CS585 prevents thrombosis in small and large vessels. (A) WT mice were administered vehicle or CS585 (0.5-6 mg/kg), followed by the induction of cremaster arteriole laser-induced injury to assess platelet and fibrin accumulation at the site of injury (n = 3 mice per condition/8 injuries per mouse). Data represent mean ± SEM. Two-way ANOVA. (B) Representative images of panel A showing platelet plug formation (green) and fibrin accumulation (red). Scale bars represent 50 μm. (C) WT mice were administered vehicle or CS585 (0.5-6 mg/kg) and the carotid artery FeCl3-induced thrombosis model was assessed (n = 5). Data represent mean ± SEM. One-way ANOVA with Dunnett correction. (D) Representative images of panel C. Scale bars represent 500 μm. (E) WT mice were administered vehicle or CS585 (6 mg/kg) and the stability of CS585 in the blood was determined by induction of the cremaster arteriole laser-induced injury to assess platelet and fibrin accumulation at the site of injury at 4 hours and 18 hours after administration (n = 3 mice per condition/8 injuries per mouse). Data represent mean ± SEM. Two-way ANOVA. (F) Representative images of panel E, showing platelet plug formation (green) and fibrin accumulation (red). Scale bars represent 50 μm. (G) WT mice were administered a single dose of vehicle or CS585 (1.5-6 mg/kg) per oral (PO) and the functional efficacy of CS585 in the blood was determined by cremaster arteriole laser-induced injury to assess platelet and fibrin accumulation at the site of injury 4 hours after administration (n = 3 mice per condition/8 injuries per mouse). Data represent mean ± SEM. Two-way ANOVA. (H) Representative images of panel G, showing platelet plug formation (green) and fibrin accumulation (red). Scale bars represent 50 μm. (I) WT mice were administered vehicle or CS585 (3 mg/kg) PO and the stability of CS585 in the blood was determined by cremaster arteriole laser-induced injury to assess platelet and fibrin accumulation at the site of injury 24, 48, and 72 hours after administration (n = 3 mice per condition/8 injuries per mouse). Data represent mean ± SEM. Two-way ANOVA. (J) Representative images of panel I, showing platelet plug formation (green) and fibrin accumulation (red). Scale bars represent 50 μm. (K) Quantification of phosphatidylserine exposure in washed platelets from WT mice IV administered with vehicle or CS585 (6 mg/kg; CS585 [IV]) and stained with annexin V, stimulated with convulxin (50 ng/mL). Washed platelets from untreated WT mice treated ex vivo with CS585 (10 μM) before annexin V staining and stimulation with convulxin (50 ng/mL) (n = 5). Quantification of the percentage of annexin V–positive events represent the levels of PS exposure. Data represent mean ± SEM. One-way ANOVA with Dunnett multiple comparisons. (L) WT mice treated with vehicle or CS585 (6 mg/kg) daily starting 24 hours before thrombus initiation. The venous thrombus mass was measured 2 days after inferior vena cava ligation (n = 5). Data represent mean ± SEM. Welch t-test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/18/10.1182_blood.2023020622/2/m_blood_bld-2023-020622-gr5il.jpeg?Expires=1765884025&Signature=SJIKSnVyx8nTHiw5VNDC0DHfBv7lclycQHgwmU48lts3i-6Np~Gd7Ae-ARlKLCUftia9x4dofsIwrYZHtJP9L-F0EmW~fzshszAzvccqKRHJ601mzt6rswkyosn7AIy-80vWXCmGXOg2Yq9zGF-rbqD0dECRUzwXED5Sjs~ERVyZlyFWIn2BqXssTT6z9HwfDOKyngCp3aoARJzSHYTiEXWzkhRXuY3QKzpMNY4rrKLy6~vCSjwXxKixpvkc5tHR-XT7dGZrfwOAz4STToJPPymTHkWoGu0bSQlA~AuNrgbYyImSTChwNEw1cc1LgV~cQXhXMqoCp7LfZsOIBey-Qg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal