In this issue of Blood, Freeman at al present the mature results of a large trial showing for the first time in a randomized comparison that the addition of 2 fractionated doses of gemtuzumab ozogamicin (GO) to intensive chemotherapy is better than 1.1 The path of the conjugated monoclonal CD33 antibody into clinical practice reads like an adventure novel, with ups and downs including temporary market withdrawal and final approval including cliff-hangers of several remaining open questions. One of the main players in the story is the dosing schedule, varying between high and low doses and single vs repetitive administration, the latter a particularly tricky role when used in combination with intensive chemotherapy.
Whereas single doses of 6 mg/m2 did not result in improved overall survival but did cause excess toxicity in the SWOG S0106 and GOELAMS/FILO 2006 trials,2,3 the use of 3 fractionated doses of 3 mg/m2 in the randomized ALFA-0701 trial led to a significant relapse-free survival benefit and the reapproval for newly diagnosed CD33+ acute myeloid leukemia (AML).4 However, a single dose of 3 mg/m2 had been used in other clinical trials by the National Cancer Research Institute (NCRI).5 In a meta-analysis of 5 randomized controlled trials that demonstrated a significant overall survival benefit, the majority of patients came from these trials using a single low dose of GO (3 mg/m2), thus reiterating the issue of optimal dosing and whether a single dose could be sufficient.6 This even led to guideline recommendations in some countries to use only a single dose of GO. In this issue of Blood, Freeman et al add the logical sequel of the GO story by comparing the dose of 3 mg/m2 GO as a single dose or repetitive administration.
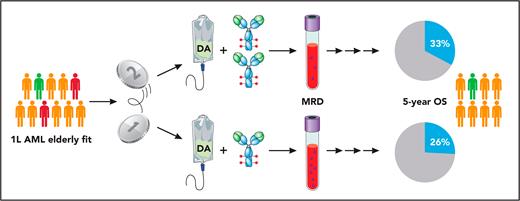
The NCRI AML18 study enrolled more than 850 fit older patients without known adverse genetic features and randomly added 1 (GO1) or 2 (GO2) doses of GO to the British version of intensive standard induction therapy containing 10 days of standard-dose cytarabine plus 3 alternating days of standard-dose daunorubicin (DA). Depending on the remission status after the initial treatment cycle, patients then received a second slightly different course of DA or were randomized again to DA plus cladribine or FLAG-Ida (fludarabine, cytarabine, idarubicin, and granulocyte colony-stimulating factor). Allogeneic hematopoietic cell transplantation was planned in eligible patients with a matched HLA donor. Multicolor flow cytometry–based centralized measurable residual disease (MRD) monitoring was performed sequentially throughout the trial.
For the first intriguing finding, the investigators showed that patients receiving 2 sequential GO doses had significantly lower MRD levels after the first cycle; likewise, significantly more patients with complete remission/complete remission with incomplete hematologic recovery in the GO2 arm reached MRD negativity. Although this difference did not translate into a survival benefit across all patients, the exclusion of randomized patients with adverse cytogenetic features that were later detected or TP53 mutations (approximately 15% of all randomized patients) led to a significant survival benefit in the remaining patients with mainly intermediate or favorable risk cytogenetics (see figure). Interestingly, this benefit was most pronounced in patients up to the age of 70 years, or with IDH mutations or with allogeneic hematopoietic cell transplantation (HCT) as postremission treatment. Similar frequencies and severities of adverse events and early deaths were detected in the treatment arms, including those related to liver toxicity and deaths attributed to infection or hemorrhage. Likewise, there was no significant difference for time of neutrophil and platelet recovery or the extent of health resource usage, with the exception of more platelet transfusions in the GO2 arm.
Design and main results of the NCRI AML18 trial. Fit older patients with newly diagnosed AML (first-line treatment, 1L) and intermediate (orange) or favorable (green) cytogenetic risk or adverse cytogenetics/TP53 mutations detected later (red) were randomized 1:1 to receive standard chemotherapy induction (standard-dose cytarabine and DA) with either 1 or 2 doses of GO. Professional illustration by Patrick Lane, ScEYEnce Studios.
Design and main results of the NCRI AML18 trial. Fit older patients with newly diagnosed AML (first-line treatment, 1L) and intermediate (orange) or favorable (green) cytogenetic risk or adverse cytogenetics/TP53 mutations detected later (red) were randomized 1:1 to receive standard chemotherapy induction (standard-dose cytarabine and DA) with either 1 or 2 doses of GO. Professional illustration by Patrick Lane, ScEYEnce Studios.
The study results demonstrate for the first time in a large prospective way that higher cumulative GO doses delivered in 2 doses increased the antileukemic efficacy and AML blast killing without excess toxicity, leading to deeper remissions and improved survival. This is most likely due to the biology of CD33 receptor kinetics. After internalization, CD33 receptors are recycled and re-expressed on the surface of the myeloblasts in approximately 72 hours. The use of a fractionated schedule every 3 days, therefore, could provide >90% saturation of re-expressed CD33 receptors and is more likely to provide consistent antileukemic activity.7 By using MRD technology, the investigators provide both a biologically plausible explanation for this dose-response relationship and more data on the clinical relevance of MRD.
The trial results do not tell us whether 3 applications would further increase chances of successful treatment, which could possibly be the next and last chapter of the GO story. But even though we cannot be sure if this sequel will ever be written, the NCRI AML18 data are clearly suggestive for a dose-response relationship and the advantage of the use of fractionated GO. This is not only relevant for patients with intermediate genetic risk who constituted the largest group in the trial but particularly for patients with favorable risk who generally gain the greatest benefit from the addition of GO to intensive chemotherapy.6 Furthermore, this study provided additional evidence against the use of GO in patients with adverse genetic features. For patients with intermediate-risk AML, there was significant antileukemic efficacy, but allogeneic HCT played an important role in increasing long-term remission.
Conflict-of-interest disclosure: C.R. has received advisory honoraria from AbbVie, Amgen, Astellas, Bristol Meyer Squibb, Jazz, Novartis, Otsuka, Pfizer, Roche, and Servier; and research funding from AbbVie, Novartis, and Pfizer.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal