In this issue of Blood, Shiloh et al uncover a novel mechanism by which dysregulated toll-like receptor (TLR) signaling drives pathologic mitogen-activated protein kinase (MAPK) pathway activity in H syndrome, a rare multisystemic histiocytic disorder. Building on these findings, they demonstrate that MAPK pathway inhibition ameliorates histiocytosis and hyperinflammation in a patient with H syndrome, providing proof of concept for future clinical studies.1
Histiocytic disorders encompass a wide range of clinical phenotypes, most of which are mediated by mononuclear phagocyte dysregulation due to somatic activating mutations in MAPK pathway genes, such as BRAF or MAP2K1.2 In contrast, H syndrome is a rare autosomal recessive disorder characterized by germ line loss-of-function mutations in SLC29A3, the gene encoding equilibrative nucleoside transporter 3 (ENT3). Tissues from patients with H syndrome demonstrate infiltration with CD68+S100+ histiocytes, often with evidence of emperipolesis. Patients also exhibit cutaneous hyperpigmentation, hypertrichosis, hepatosplenomegaly, hearing loss, heart anomalies, hypogonadism, hyperglycemia, and hyperinflammation.3
Through immunohistochemical analysis of histiocytic lesions from patients with H syndrome, Shiloh et al find nearly universal MAPK pathway activation, as revealed by high levels of phosphorylated extracellular signal-regulated kinase (pERK). Nevertheless, exome sequencing of these lesions reveals no evidence of somatic mutations, including in any MAPK pathway genes. Thus, these studies demonstrate that germ line SLC29A3 mutations in themselves are sufficient to drive the formation of histiocytic lesions.
To understand this unanticipated association, Shiloh et al perform RNA sequencing of monocytes from patients with H syndrome vs monocytes from healthy controls. Through gene set enrichment analysis, they demonstrate significant enrichment for genes associated with TLR signaling in H syndrome monocytes. TLRs function by recognizing pathogen-associated molecular patterns, leading to downstream signaling, including activation of the MAPK pathway. Two members of this family, TLR7 and TLR8, are expressed in monocytes and localized to the lysosomal membrane, where they bind nucleoside ligands.4 ENT3 is a lysosomal protein responsible for carrying nucleosides across the lysosomal membrane and into the cytoplasm. In mononuclear phagocytes, nucleosides accumulate in the lysosome following target cell phagocytosis, and their export is essential for preserving lysosomal activity and overall phagocyte homeostasis.5 The authors hypothesize that nucleosides might accumulate in the lysosomes of H syndrome monocytes due to dysfunctional ENT3 protein, and that this would then activate the MAPK pathway via TLR7/8. Indeed, through a series of in vitro studies, the authors reveal that TLR7/8 activation induces pERK in healthy donor monocytes and that this activation can be ablated with a TLR8 inhibitor.
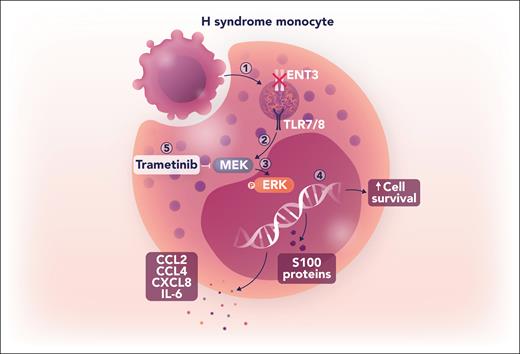
The transcriptional data from H syndrome monocytes also reveal upregulation of proinflammatory cytokine genes, S100 genes, and the prosurvival BCL2A1 gene. Shiloh et al validate these findings by demonstrating increased cytokine secretion from H syndrome monocytes in vitro, which is attenuated following treatment with a TLR8 or an MAPK pathway inhibitor. On the basis of these results, they go on to administer the MEK inhibitor trametinib to a patient with H syndrome with histiocytic lesions that were refractory to conventional therapies. Remarkably, the patient demonstrates complete and sustained tumor resolution with trametinib monotherapy, with concomitant downregulation of proinflammatory cytokine genes and BCL2A1 expression in monocytes. Taken together, these data unveil a mechanism whereby ENT3 dysfunction leads to nucleoside accumulation in the lysosomes of H syndrome monocytes. This nucleoside accumulation activates TLR7/8, leading to downstream MAPK signaling that, in turn, induces a proinflammatory transcriptional program and facilitates the persistence of histiocytic lesions, all of which are reversible with MAPK inhibition (see figure).
Loss of function of ENT3 activates MAPK signaling through a noncanonical pathway. (1) Following target cell phagocytosis in an H syndrome monocyte, dysfunctional ENT3 prevents translocation of degraded nucleosides into the cytoplasm, resulting in lysosomal accumulation. (2) Accumulation of nucleosides activates TLR7/8, leading to downstream signaling. (3) TLR7/8 signaling stimulates ERK phosphorylation in an MEK-dependent manner. (4) Increased pERK activity alters the transcriptional program to increase production of proinflammatory cytokines, S100 proteins, and the prosurvival protein BCL2A1. (5) Inhibition of MEK signaling with trametinib effectively attenuates inflammatory cytokine production and resolves histiocytic lesions in a patient with H syndrome. IL, interleukin. Professional illustration by Somersault18:24.
Loss of function of ENT3 activates MAPK signaling through a noncanonical pathway. (1) Following target cell phagocytosis in an H syndrome monocyte, dysfunctional ENT3 prevents translocation of degraded nucleosides into the cytoplasm, resulting in lysosomal accumulation. (2) Accumulation of nucleosides activates TLR7/8, leading to downstream signaling. (3) TLR7/8 signaling stimulates ERK phosphorylation in an MEK-dependent manner. (4) Increased pERK activity alters the transcriptional program to increase production of proinflammatory cytokines, S100 proteins, and the prosurvival protein BCL2A1. (5) Inhibition of MEK signaling with trametinib effectively attenuates inflammatory cytokine production and resolves histiocytic lesions in a patient with H syndrome. IL, interleukin. Professional illustration by Somersault18:24.
Aberrant MAPK activity is a common feature of histiocytic diseases. In Langerhans cell histiocytosis (LCH) and Erdheim-Chester disease (ECD), somatic mutations in BRAF or other components of the signaling cascade typically drive increased pathway activity.2 Despite biochemical evidence of MAPK activation, H syndrome is unique in its lack of activating pathway mutations, and the work by Shiloh et al is notable in providing a mechanistic link between ENT3 dysfunction and activation of the MAPK pathway. Using a variety of complementary approaches, the authors provide compelling evidence supporting a role for TLR7/8 in activating MAPK and driving disease sequelae.
However, several key questions remain. In particular, although the authors demonstrate that TLR8 inhibition attenuates pERK, the precise mechanism linking TLR signaling and the MAPK pathway has yet to be elucidated. It has been shown in other contexts that on ligand engagement, the cytoplasmic domains of TLRs recruit a complex of adaptor proteins, including MyD88, TRAF6, and TAK1. This aggregation activates TAK1, which then phosphorylates MEK.6 Further studies are needed to determine whether this same signaling cascade is responsible for MAPK pathway activation in H syndrome. In addition, a recent study reports that mice deficient in Slc29a3 develop histiocytic lesions, and that these lesions are absent in mice with a simultaneous Tlr7 deficiency. Although these findings are consistent with those of Shiloh et al, there is not an increase in pERK in the tissues of Slc29a3-deficient mice,7 suggesting that MAPK-independent pathways downstream of TLR signaling may contribute to H syndrome pathogenesis. Future work to define these pathways may provide additional mechanistic insights and reveal other therapeutic vulnerabilities in this disease.
Given the frequency of MAPK pathway activation in LCH and ECD, there has been considerable interest in using MAPK inhibitors to treat these diseases.8 Although it has been shown that patients with activating MAPK pathway mutations benefit most from this therapeutic strategy, data from a trial using the MEK inhibitor cobimetinib demonstrate clinical responses even in patients lacking such mutations,9 further supporting a role for MAPK inhibitors in the treatment of H syndrome. Consistent with this notion, Shiloh et al report tumor resolution in a patient treated with trametinib, confirming the potential for MAPK inhibition to produce significant and durable disease responses. However, there is little consensus in other histiocytic diseases regarding the appropriate duration of therapy, and disease recurrence after discontinuation has been a significant limitation requiring additional investigation.8 Nevertheless, the work by Shiloh et al demonstrates the power of studying rare disorders to provide novel insights into health and disease. They present convincing preclinical data justifying further investigation into the utility of MAPK pathway inhibitors in the treatment of patients with H syndrome.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal