In this issue of Blood, Denton et al1 show that luspatercept induces iron redistribution by reducing splenic iron without changing liver iron content in patients with β-thalassemia (β-thal). This challenges the understanding that the iron content of the liver reflects total body iron and suggests that magnetic resonance imaging (MRI) scans should ideally include the liver and spleen to adequately monitor iron status in patients with iron-loading anemias treated with an erythroid maturation agent.
Ineffective erythropoiesis (IE) implies an expansion of early-stage erythroid progenitors and apoptosis of their late-stage counterparts. IE is the key defect in the so-called iron-loading anemias, which include both inherited (eg, β-thal) and acquired (eg, low-risk myelodysplastic syndromes [MDSs]) disorders.2 In iron-loading anemias, the iron overload is related to chronic transfusions and/or increased iron absorption due to hepcidin suppression by factors released by disturbed erythroid progenitors, and substantially contributes to the disease’s burden. Targeted therapy for IE has been a long-standing goal, recently addressed by the development of luspatercept, a first-in-class erythroid maturation agent. Luspatercept is a recombinant fusion protein that binds transforming growth factor-β (TGF-β) superfamily ligands, thereby preventing their interaction with type II TGF-β receptors. This counteracts the constitutive SMAD2/3 signaling, which inhibits red blood cell maturation in IE, ultimately promoting erythropoiesis. Unexpectedly, despite successes in improving anemia and reducing transfusion burden in either β-thal or low-risk MDS,3,4 luspatercept did not reduce the liver iron concentration (LIC) estimated by MRI, which is now considered the best available proxy of total body iron. Using an internal protocol, Denton et al measured spleen iron concentration and content (ie, concentration × volume) in addition to usual liver iron parameters in patients with β-thal before and after luspatercept therapy (median time of drug exposure, 30.9 months). Although confirming that LIC remained unchanged, the authors observed a substantial decrease in spleen iron content, with a Cohen d coefficient (the difference between 2 means divided by the SD, which estimates the effect size) ≥1.0, which means a large to very large effect. This suggests that luspatercept may induce a redistribution of iron, ultimately leading to a decrease of total body content, which is not captured by the current measurement of LIC. This study has important limitations. First, the number of patients with β-thal examined (11 transfusion dependent and 4 non–transfusion dependent) was small. Second, the MRI-based estimate of spleen iron might be not as precise as liver iron, because of the obvious inability to validate the method through biopsy and direct iron quantification. Nevertheless, if confirmed, the observation by Denton et al implies a key practical message about the limits of measuring just LIC in complex iron overload disorders associated with IE, like β-thal or low-risk MDS, especially when performed to monitor the efficacy of treatments. On the other hand, there is an urgent need of sharing standardized protocols for measuring spleen iron by MRI, which still vary considerably even among referral centers and should be validated as well as possible.
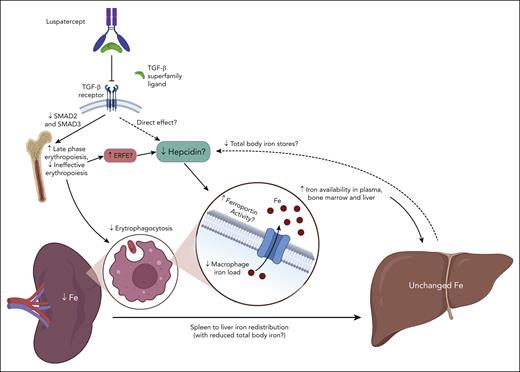
From a mechanistic perspective, the intriguing observation by Denton et al raises more questions than answers. Reduced spleen iron content in luspatercept-treated patients could merely represent the effect of decreased transfusions and the ensuing reduced erythrophagocytosis. However, it was observed also in non–transfusion-dependent patients with β-thal. An improved response to iron chelation therapy was also deemed unlikely to explain the magnitude of the effect. On the basis of data from a substudy of the BELIEVE trial,5 where hepcidin decreased by 53% and erythroferrone (ERFE) increased by 51% in transfusion-dependent patients with β-thal after 48 weeks of luspatercept therapy, a mechanism could be hypothesized (see figure). This is partially counterintuitive and not entirely consistent with data from initial mouse models treated with RAP-536 (the murine counterpart of luspatercept). With the improvement of IE, one would expect a decrease of hepcidin-suppressing signals by disturbed erythroid progenitors. Indeed, thalassemic mice treated with RAP-536 showed an increased (rather than decreased) hepcidin expression,6 whereas a decreased spleen iron content was observed, consistently with human counterparts. This apparent discrepancy in hepcidin behavior may be explained by the different experimental time courses (8 weeks in animals vs 48 weeks in humans), and by complex dynamic changes of iron metabolism that cannot be captured by single-point measurements. Like any hormone, hepcidin is subjected to a variety of stimuli and counterregulatory responses that may vary over time and make the interpretation of circulating levels possible only in the context of the whole clinical picture.7 In iron-loading anemias, hepcidin levels are generally higher than normal but relatively suppressed compared with the increased iron stores. In the long run, luspatercept treatment may decrease hepcidin by decreasing total body iron stores, and/or by a direct suppressing effect because hepcidin has been shown to be stimulated in vitro by TGF-β1 in mouse and human hepatocytes.8 As mentioned above, increased ERFE levels possibly related to increasingly maturing erythroblasts may also contribute to relative hepcidin decrease during luspatercept treatment.5,9 However, hepcidin regulation by erythropoietic signals involves more than a single factor, and it is still not completely understood, especially in disorders characterized by IE.2 Similarly, TGF-β signaling looks like one of the most complex and pleiotropic systems in the body (recently extensively reviewed by Massague et al10); thus, luspatercept effects on iron metabolism may extend beyond the current and relatively simplified view.
Luspatercept decreases spleen iron content without substantial changes in liver iron content. Hypothetical mechanism(s) proposed by Denton et al,1 which need confirmation by future studies. By improving late-stage erythropoiesis, luspatercept may negatively modulate hepcidin via multiple mechanisms, including the following: (1) increased release of mediators by erythroid progenitors (ERFE? others still unidentified?); (2) direct interference on signaling pathways activating hepcidin, which can also involve TGF-β receptor signaling; and (3) reduction of total body iron stores, to which hepcidin production is generally proportional. These dynamic changes, along with decreased erythrophagocytosis due to reduction of transfusion burden and IE, may promote ferroportin activity in splenic macrophages, leading to increased iron egress from the spleen, as well as increased iron availability in plasma, bone marrow, and liver, favoring iron redistribution and increased iron chelation efficacy. Question marks denote mechanisms that need confirmation. Figure created with BioRender.com.
Luspatercept decreases spleen iron content without substantial changes in liver iron content. Hypothetical mechanism(s) proposed by Denton et al,1 which need confirmation by future studies. By improving late-stage erythropoiesis, luspatercept may negatively modulate hepcidin via multiple mechanisms, including the following: (1) increased release of mediators by erythroid progenitors (ERFE? others still unidentified?); (2) direct interference on signaling pathways activating hepcidin, which can also involve TGF-β receptor signaling; and (3) reduction of total body iron stores, to which hepcidin production is generally proportional. These dynamic changes, along with decreased erythrophagocytosis due to reduction of transfusion burden and IE, may promote ferroportin activity in splenic macrophages, leading to increased iron egress from the spleen, as well as increased iron availability in plasma, bone marrow, and liver, favoring iron redistribution and increased iron chelation efficacy. Question marks denote mechanisms that need confirmation. Figure created with BioRender.com.
Overall, the novel findings by Denton et al raise several possible clinical implications that warrant further studies. In particular, it should be investigated whether luspatercept-mediated iron redistribution may involve other organs more directly affected by iron toxicity than the spleen, like the heart and pancreas. Similarly, a deeper understanding on the effects of iron redistribution on the cheatable iron pool may provide insights to improve timing and combination of therapeutic options in iron-loading anemias due to IE.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal