Abstract
Understanding the genetic alterations, disrupted signaling pathways, and hijacked mechanisms in oncogene-transformed hematologic cells is critical for the development of effective and durable treatment strategies against liquid tumors. In this review, we focus on the specific involvement of the Hedgehog (HH)/GLI pathway in the manifestation and initiation of various cancer features in hematologic malignancies, including multiple myeloma, T- and B-cell lymphomas, and lymphoid and myeloid leukemias. By reviewing canonical and noncanonical, Smoothened-independent HH/GLI signaling and summarizing preclinical in vitro and in vivo studies in hematologic malignancies, we elucidate common molecular mechanisms by which HH/GLI signaling controls key oncogenic processes and cancer hallmarks such as cell proliferation, cancer stem cell fate, genomic instability, microenvironment remodeling, and cell survival. We also summarize current clinical trials with HH inhibitors and discuss successes and challenges, as well as opportunities for future combined therapeutic approaches. By providing a bird's eye view of the role of HH/GLI signaling in liquid tumors, we suggest that a comprehensive understanding of the general oncogenic effects of HH/GLI signaling on the formation of cancer hallmarks is essential to identify critical vulnerabilities within tumor cells and their supporting remodeled microenvironment, paving the way for the development of novel and efficient personalized combination therapies for hematologic malignancies.
Hedgehog (HH)/GLI signaling in oncology: success, failure, and challenges
HH/GLI signaling is a prime example of translating basic scientific knowledge into medical applications. More than 30 years after the discovery of the HH pathway in fruit flies,1 vismodegib was approved in 2012 by the US Food and Drug Administration (FDA) as the first HH inhibitor for the treatment of HH-driven advanced and metastatic basal cell carcinoma.2-4 However, the last 10 years have seen many setbacks in clinical trials, and it was not until 2018 that the next breakthrough was reported. Glasdegib, like vismodegib, a specific inhibitor of the essential HH effector Smoothened (SMO), significantly improves survival in patients with acute myeloid leukemia (AML) in combination with low-dose chemotherapy.5 This landmark study triggered a series of phase 1/2 trials in hemato-oncology patients to investigate the therapeutic efficacy of SMO inhibitors (SMOis), primarily in combination with chemotherapeutics, hypomethylating agents (HMAs), or proapoptotic drugs (for details, see “Hopes and fails: HH/GLI targeting in clinical trials”). The results indicate that only a few combination treatments provide a benefit to patients. Although this underscores the therapeutic potential of SMOis for the treatment of selected hematologic malignancies, the many failed trials highlight the need for a detailed understanding of oncogenic HH signaling in the hematopoietic system.
The aim of this review is to provide a comprehensive overview of the numerous publications on the topic of HH signaling in hematologic malignancies, to distill from this wealth of data a bird’s eye view of common and recurrent oncogenic modes of action resulting from uncontrolled HH signaling across different tumor entities and provide a basis for developing new ideas and strategies for combination treatments.
HH/GLI signaling and its role in normal hematopoiesis
The molecular steps of HH/GLI signaling in mammalian adherent cells, with the primary cilium acting as a central hub for activation,6,7 are well-understood (for detailed reviews, see8-11). In nonadherent hematopoietic cells, traditionally believed to lack primary cilia, HH/GLI signaling remains enigmatic. However, cilia-like structures have been observed in hematopoietic and leukemic cells, suggesting their involvement in HH/GLI signaling.12-14 In addition, cilia-associated factors are localized at the immunological synapse, which could serve as a site of HH/GLI activation.15-18
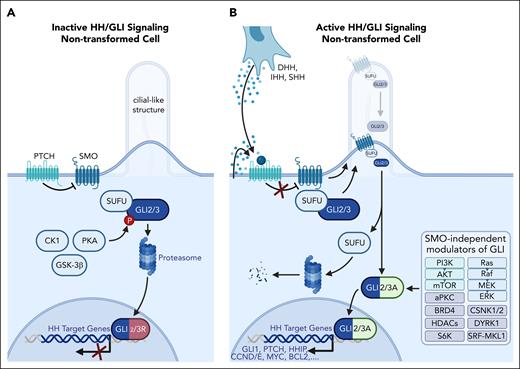
In the absence of HH ligands, SMO-dependent HH/GLI signaling is inhibited by Patched (PTCH) and Suppressor of Fused (SUFU), resulting in the generation of GLI repressor forms or degradation of GLI proteins (Figure 1A). Upon binding of HH ligands to PTCH, SMO translocates to the primary cilium, where it induces the dissociation of GLI from SUFU to promote the generation of GLI activators that induce HH target gene transcription. Notably, GLI activity is also induced by SMO-independent effectors, including phosphatidylinositol 3-kinase (PI3K)/AKT serine/threonine kinase (AKT)/mechanistic target of rapamycin kinase (mTOR), Ras/Raf/MEK/extracellular signal-regulated kinase (ERK), casein kinases (CSNK1/2), DYRK1, atypical protein kinase (PKC), SRF-MKL1, BRD4, S6K, and class-I histone deacetylases (Figure 1B).19-38
HH/GLI signaling in nontransformed hematologic cells. (A) In the absence of HH ligand, HH/GLI signaling is actively repressed by the formation of GLI repressor transcription factors (GLI2/3R). In its unliganded form, the HH receptor PTCH prevents the entry of the essential HH effector SMO into cilia-like structures. The absence of SMO allows phosphorylation of SUFU-bound GLI transcription factors (GLI2/3) via PKA, CK1, and GSK-3β kinases. Phosphorylated GLI2/3 is partially degraded by the proteasome, yielding C-terminally truncated GLI repressor proteins that translocate to the nucleus to repress target gene expression. (B) Autocrine or paracrine HH signaling is initiated by binding of the HH ligand to its receptor, PTCH, resulting in shuttling and activation of SMO. Activated SMO prevents proteasomal degradation of GLI2/3 and promotes the release of GLI from inhibitory SUFU, resulting in GLI2/3 activator forms (GLI2/3A) and subsequent induction of HH target genes, including GLI1, amplifying the GLI activator signal. In addition, GLI activation can occur in a SMO-independent manner via PI3K/AKT/mTOR (light green) and Ras/Raf/MEK/ERK (light blue) signaling, or in a more indirect manner via cofactors and DNA modifiers, including casein kinases (CSNK1, CSNK2), DYRK1, atypical PKC, SRF-MKL1, BRD4, S6K, and class-I histone deacetylases (gray).
HH/GLI signaling in nontransformed hematologic cells. (A) In the absence of HH ligand, HH/GLI signaling is actively repressed by the formation of GLI repressor transcription factors (GLI2/3R). In its unliganded form, the HH receptor PTCH prevents the entry of the essential HH effector SMO into cilia-like structures. The absence of SMO allows phosphorylation of SUFU-bound GLI transcription factors (GLI2/3) via PKA, CK1, and GSK-3β kinases. Phosphorylated GLI2/3 is partially degraded by the proteasome, yielding C-terminally truncated GLI repressor proteins that translocate to the nucleus to repress target gene expression. (B) Autocrine or paracrine HH signaling is initiated by binding of the HH ligand to its receptor, PTCH, resulting in shuttling and activation of SMO. Activated SMO prevents proteasomal degradation of GLI2/3 and promotes the release of GLI from inhibitory SUFU, resulting in GLI2/3 activator forms (GLI2/3A) and subsequent induction of HH target genes, including GLI1, amplifying the GLI activator signal. In addition, GLI activation can occur in a SMO-independent manner via PI3K/AKT/mTOR (light green) and Ras/Raf/MEK/ERK (light blue) signaling, or in a more indirect manner via cofactors and DNA modifiers, including casein kinases (CSNK1, CSNK2), DYRK1, atypical PKC, SRF-MKL1, BRD4, S6K, and class-I histone deacetylases (gray).
During normal hematopoiesis, HH/GLI signaling has pleiotropic effects. For example, treatment of human hematopoietic stem and progenitor cells (HSPCs) increases their in vitro proliferation and in vivo repopulation capacity.39,40 However, these findings are challenged by studies using mouse models analyzing the contribution of PTCH and SMO to normal hematopoiesis. Although adult Ptch1+/− mice showed an expansion of hematopoietic progenitor cells in the bone marrow (BM) under steady-state conditions and increased proliferation and exhaustion of hematopoietic stem cells (HSCs) under hematopoietic stress,41,42 the transplantation of Ptch1-deficient HSPCs into Ptch1+/+ recipients revealed that PTCH1 function in HSPC maintenance is not intrinsic to HSPCs but provided by the supportive microenvironment.43,44 Similarly, tissue-specific knockout of Smo suggested that endothelial but not hematopoietic SMO expression is essential for HSPC function.45,46 In the context of T- and B-cell differentiation, HH ligands regulate balanced effector cell production and receptor selection at distinct stages of differentiation.47-49 Similarly, T-cell–specific GLI2A overexpression led to impaired T-cell activation, signaling, and proliferation.50 Focusing on specific T-cell subtypes, it was shown that SHH signaling promotes γδ T-cell development and differentiation into γδ NK T-cells and ensures their subsequent survival and proliferation.51,52 In Dhh-deficient mice, the lack of desert hedgehog (DHH) in the BM resulted in increased megakaryocyte-erythroid hematopoietic progenitor cell numbers and erythrocyte production.53
In conclusion, unraveling the involvement and control of HH/GLI signaling in hematopoietic cells remains challenging because of its context-dependent nature and the broad spectrum of (SMO-independent) signal-integrating processes. Rigorous approaches are needed to validate findings and ensure human relevance for the development of immune-modifying and anticancer drugs targeting HH/GLI.
HH/GLI-driven cancer hallmarks in hematologic malignancies
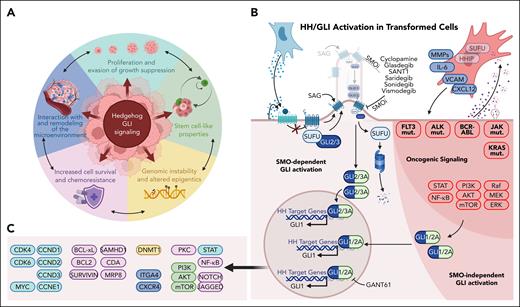
More than 20 years ago, Hanahan and Weinberg introduced the cancer hallmark concept by generalizing and simplifying the steps and mechanisms that contribute to tumor development and progression. Since then, the original 6 hallmarks (self-sufficiency in growth signals, insensitivity to antigrowth signals, metastasis, unlimited replicative potential, sustained angiogenesis, and evasion of cell death) have been refined, resulting in the currently proclaimed 14 cancer hallmark capabilities of cancer cells, the immune microenvironment, and the microbiome.54-56 These revisions reflect not only the scientific knowledge gained over time but also the now appreciated diversity among tumor entities and their distinct evolutionary trajectories. The hallmarks concept provides a powerful framework for innovative therapeutic strategies that target multiple vulnerabilities. In the following review, we will discuss how HH/GLI signaling influences and/or drives the most prominent hallmarks of liquid cancers (Figure 2).
Molecular control of cancer hallmarks by HH/GLI signaling in hematologic malignancies. (A) HH/GLI signaling in hematologic malignancies plays a key role in the establishment of cancer hallmarks. These illustrate the oncogenic mechanisms driven by HH/GLI in fluid cancers and represent common therapeutic vulnerabilities for the rational design of more effective and durable combination therapies. (B) Activation and intricate interactions between HH/GLI and other oncogenic driver pathways resulting in enhanced transforming activity through increased SMO-dependent and SMO-independent oncogenic GLI activation. (C) HH/GLI target genes and their association as causal factors in the establishment of cancer hallmarks determined by HH/GLI signaling (the color code of target gene clusters corresponds to the color code of cancer hallmarks in panel A).
Molecular control of cancer hallmarks by HH/GLI signaling in hematologic malignancies. (A) HH/GLI signaling in hematologic malignancies plays a key role in the establishment of cancer hallmarks. These illustrate the oncogenic mechanisms driven by HH/GLI in fluid cancers and represent common therapeutic vulnerabilities for the rational design of more effective and durable combination therapies. (B) Activation and intricate interactions between HH/GLI and other oncogenic driver pathways resulting in enhanced transforming activity through increased SMO-dependent and SMO-independent oncogenic GLI activation. (C) HH/GLI target genes and their association as causal factors in the establishment of cancer hallmarks determined by HH/GLI signaling (the color code of target gene clusters corresponds to the color code of cancer hallmarks in panel A).
Disrupting HH/GLI signaling to target proliferation and evasion of growth suppression
Tumorigenesis is directly linked to uncontrolled proliferation, which is achieved through the self-sustaining production of proliferative signals such as HH/GLI57-59 and the avoidance of growth-inhibitory cues. To understand this escape from homeostatic constraints, specifically in hematologic malignancies, it is crucial to differentiate between the primary tumor mass and tumor-fueling cancer stem cell-like (CSC-like) populations, which rely on partially different pathways to regulate their cell cycle progression.
For example, multiple myeloma (MM) is characterized by the accumulation of less-differentiated CD19+CD81+, more-differentiated CD19−CD81+, and/or fully differentiated CD19−CD81− plasma cells within the BM containing CD138− MM CSCs.60 Consistent with increased SMO expression in and SHH-induced proliferation of MM CSCs,61,62 the less differentiated the malignant plasma cell population, the higher the expression of HH genes.63 In addition, high expression of HH genes in MM plasma cells correlates with increased genomic instability and poor patient prognosis.64 However, the extent to which HH signaling contributes to bulk MM proliferation is unclear. Although initial studies suggested that bulk MM cells proliferate only partially in response to SHH,61 subsequent analyses showed that most MM cells and their surrounding BM stromal cells produce and secrete SHH, thereby supporting general MM cell proliferation.65,66 In addition to altered HH gene expression, GLI1 activity and in vivo tumorigenicity are increased in MM because of RNA editing of GLI1 by ADAR1, resulting in decreased binding of the GLI-inhibitor SUFU to GLI1.67 Accordingly, the GLI antagonist GANT61 reduced MM cell proliferation due to G0/G1 cell cycle arrest.68-71 Possibly due to differences in SMO-dependent and SMO-independent HH/GLI signaling in MM, application of the SMOi sonidegib resulted in G2/M phase arrest.72
High levels of HH core components (HH ligands, PTCH, SMO, GLI1, GLI2, and GLI3) are associated with poor prognosis in several types of Hodgkin and non-Hodgkin lymphomas, including natural killer/T-cell lymphoma (NKTCL), T-cell lymphoblastic lymphoma, anaplastic lymphoma kinase–positive (ALK+) anaplastic large cell lymphoma (ALCL), diffuse large B-cell lymphoma (DLBCL), splenic marginal zone lymphoma, mantle cell lymphoma (MCL), Burkitt lymphoma, chronic lymphocytic leukemia (CLL), and many others. Although increased GLI1 and SMO expression was associated with gene amplification, chromosomal aneuploidy, or point mutations in several B-cell lymphomas, GLI1 and SHH expression were also directly upregulated by ALK/PI3K/AKT activity in ALCL.73-85 Inhibition of SMO resulted in decreased proliferation in vitro and/or in vivo and decreased expression of GLI targets CCND1, CCND2, and/or CCNE1 in NKTCL,86,87 T-cell lymphoblastic lymphoma,74 germinal center type but not activated B-cell type DLBCL,88 Myc driven murine B-cell lymphoma,65 ALCL,75 and some but not all MCL78 cells. Furthermore, treatment of B-cell lymphoma cells with recombinant or stromal cell–derived SHH confirmed HH signaling–induced cell cycle progression.65,78 Nonresponsive MCL cells were shown to exhibit SMO-independent GLI activity and proliferation.78
Two independent exome sequencing studies identified single nucleotide variants in HH-related genes (SMO, GLI1, GLI3, PTCH1, PTCH2, and SHH) in samples from patients with acute lymphoblastic leukemia (ALL) that may contribute to increased HH gene expression and signaling in around 20% of T-ALL samples.89-92 In addition, GLI1, SHH, and IHH expression are positively regulated by the ALL-associated transcription factors GATA1, GATA2, and possibly NF-κB2. Importantly, chemical and genetic targeting of SMO or GLI1 reduced the proliferation of ALL cell lines.91 Under serum starvation mimicking nutrient deprivation during rapid tumor growth, ALL cells were shown to downregulate AMP-activated protein kinase (AMPK) signaling, which increased GLI1 expression and activity. Furthermore, GLI inhibition resulted in decreased tumor cell growth and viability.93,94 Consistent with these in vitro and ex vivo observations, ectopic expression of SHH and IHH in JAK3-mutant T-ALL cells resulted in increased Gli1, Ptch1, Ptch2, Bcl-2, Ccnd1, and Ccnd2 expression levels and a growth advantage over coinjected and competing control T-ALL cells in leukemic mice.91
Compared with healthy controls, increased expression of SHH, DHH, SMO, and GLI1 has also been observed in samples from patients with myelodysplastic syndrome (MDS).95-98 Elevated SMO and GLI1 expression correlated with DNMT1 expression and DNA methylation activity, poor prognosis, and, consistent with data from an MDS mouse model expressing constitutively active SMO (SmoM2), also with the transition of patients from MDS to AML blast crisis.96-99 Although the plant-derived SMOi jervine reduced proliferation and S-phase entry of MUTZ1 MDS cells,95 the clinically approved SMOi glasdegib did not affect the proliferation of MDS-L cells. However, abrogation of both SMO-dependent and SMO-independent HH/GLI signaling in MDS-L or MUTZ1 cells resulted in impaired cell growth, indicating disease heterogeneity with respect to HH signaling among MDS cell lines and patients.96,97
Core components of HH signaling are differentially expressed among patients with AML, with GLI1 overexpression being associated with poor patient prognosis and GLI2 activity being associated with FLT3 mutations, high leukemic stem cell (LSC) frequency, and reduced patient survival.100-108 In contrast, GLI3 is typically methylated and not transcribed in most patients with AML. However, when GLI3 is expressed in AML cells, it acts as an HH repressor that antagonizes AKT signaling and redirects HH signaling to depend on SMO.104 AML studies of HH ligand expression have yielded conflicting results, suggesting either that leukemic cells produce SHH and/or IHH mRNA100-102,104 or that HH ligand production and secretion, primarily DHH, is restricted to AML BM stromal cells.103 Analysis of cells derived from patients with AML revealed a predominance of SMO-independent over SMO-dependent HH/GLI signaling. Although neither SMOi, recombinant HHIP, nor anti-HH ligand blocking antibody treatment resulted in profound antiproliferative effects, GLI targeting abrogated PI3K/AKT signaling and downstream cyclin dependent kinase (CDK)4, CDK6, CCND1, CCND2, and CCND3-mediated cell cycle progression.101,104,107,109-112 It is important to note, however, that response rates varied dramatically among cells harboring different oncogenic mutations. Consistent with these observations, triple-inhibition of FLT3, PI3K, and GLI (but not SMO) signaling reduced FLT3-ITD+ AML cell proliferation.110 Furthermore, mice with combined FLT3-ITD and SmoM2 activation exhibited increased STAT5 signaling, expanded myeloid progenitors, and accelerated AML disease progression. Of note, combined SMO and FLT3-ITD inhibition curbed leukemic cell growth and prolonged the survival of leukemic mice.111
In chronic myeloid leukemia (CML), BCR-ABL proto-oncogene 1 (ABL)–transformed LSCs and bulk BM cells upregulate HH core components and proliferate in an HH signaling–dependent manner.42,113,114 Expression of constitutively active SMO in leukemic mice, addition of recombinant SHH to LSCs ex vivo, or overexpression of SHH in vitro resulted in increased LSC/CML cell proliferation. Consistently, blocking HH signaling resulted in downregulation of MYC and cell cycle arrest.42,113-116 Similarly, the BM of patients with JAK2V617F myeloproliferative neoplasm (MPN) expresses increased levels of DHH and PTCH1. However, clear evidence for altered HH-dependent cell proliferation in MPNs is limited to the HH signaling suppressor PTCH2. Loss of PTCH2 expression, observed in 70% of patients with MPN, triggered increased HSPC cycle activity in a JAK2V617F-driven MPN mouse model. Importantly, these elevated proliferation rates led to the exhaustion of PTCH2-deficient LSCs over time.117
Disrupting HH/GLI signaling to target stem cell–like properties
A key feature of hematologic malignancies is their hierarchically organized tumor cell production, which originates from transformed, disease-driving, resistant, and relapse-causing HSPCs. Targeted elimination of this highly malignant but rare cell population by, for example, HH inhibition may represent a promising, potentially curative therapeutic approach.
In T- and B-ALL cells, the proportion of stem cell–like colony-forming progenitors was reduced upon HH/SMO/GLI inhibition.94,118 SMOi-pretreatment of B-ALL cells transplanted into immunosuppressed mice prolonged survival, indicating impaired establishment or support of the main tumor cell population. SMO inhibition severely impaired LSC self-renewal, as demonstrated by serial transplantation experiments.118 Interestingly, when GLI1-deficient T-ALL cells were transplanted into immunosuppressed mice, engraftment was reduced because of lower AKT and CXCR4 signaling, suggesting that GLI1 contributes to ALL cell homing to and migration within stemness-inducing niches.94
Similar observations have been made in MM, where the addition of recombinant SHH or overexpression of a constitutively active SMO resulted in increased clonogenicity of MM cells, and knockdown, or pharmacological inhibition of SMO or GLI1 reduced their clonal growth rates. Furthermore, GLI1 expression and activity correlated with increased CSC self-renewal.61,67,70,119
Although MDS and AML are considered role models for CSC-related diseases,120 there are only a few studies that tested for altered HSPC number and function upon HH pathway manipulation. In an MDS mouse model, overexpression of SmoM2 led to the activation of self-renewal pathways, resulting in increased HSPC colony formation in vitro and increased HSC serial transplantation efficiency. Notably, inhibition of GLI1, but not SMO, reduced human MDS cell colony growth.96 In AML, SMOi-induced inhibition of clonal growth was restricted to FLT3-ITD+ and AML-ETO+ cell lines, whereas GANT61 more broadly blocked clonal growth of various AML cell lines (eg, FLT-3-ITD+, AML1-ETO+, c-Kit-, JAK3-, or KRAS-mutated).109,112 The inhibitory effect of GANT61 was further enhanced by combination with BET bromodomain, or PI3K, and multityrosine kinase inhibitors, such as sunitinib.109,110 In vivo treatment of xenografts from patients with primary AML with glasdegib did not change the initial tumor burden, though after serial transplantation, secondary recipients did not develop AML because of the loss of LSCs. Importantly, glasdegib did not affect healthy HSC function.121
In the PTCH2 KO MPN mouse model, HSPCs were mobilized from the BM to the periphery, resulting in compensating increased HSC cell cycling within the BM. However, PTCH2 inactivation reduced colony-forming unit (CFU) replating and in vivo repopulation efficiency.117 HH signaling is also essential for the long-term maintenance of LSCs in CML, as SMOi treatment resulted in reduced colony growth, which was further decreased by tyrosine kinase inhibitors.42,113,122,123 BCR-ABL+ fetal liver cells deficient in SMO also failed to expand in mice, whereas BCR-ABL+ PTCH1-deleted fetal liver cells rapidly engrafted and established a CML-like disease sensitive to SMOi treatment. In addition, the self-renewal capacity of LSCs was decreased by genetic and chemical perturbations of SMO function.42,113,122 Of note, CML xenografts simultaneously treated with sonidegib and nilotinib had a significantly reduced tumor burden, prolonged overall survival, and reduced numbers of retransplantable LSCs.123
Disrupting HH/GLI signaling to target interaction with and remodeling of the tumor microenvironment
Tumor cell intrinsic and extrinsic HH signaling has been described to be altered in hematologic malignancies. However, only little is known about its effect on the composition and function of supportive tumor niches. For example, CLL cells directly manipulate their environment by secreting exosomes that are taken up by stromal cells, resulting in upregulation of c-FOS and ATM and downregulation of SUFU,124 yet the functional consequences are unknown. In MCL, SMO-mediated signaling is required for stromal cells to provide MCL cell–supporting interleukin-6 and CXCL12 signals and to present vascular cell adhesion molecule (VCAM)-1 on their cell surface for tumor cell retention. Consistent with this observation, SMOi treatment of MCL cells reduced the expression of the VCAM-1 counterreceptor very lare activation protein 4 receptor, alpha 4 subunit (VLA-4), focal adhesion kinase (FAK), and PAXILLIN, resulting in curbed migration on and adhesion to stromal cells.125
In MM, stromal cells are reprogrammed by MM cell-derived SHH to produce CYP26, an enzyme crucial for maintaining a retinoic acid-low microenvironment that prevents MM cell differentiation and promotes bortezomib resistance.126 However, this finding is challenged by a study suggesting that BM stromal cells derived from patients with MM lack SMO expression and thus canonical HH signaling.66 Nevertheless, MM cell-derived SHH also converted macrophages into proangiogenic MM-associated macrophages that induce chemoresistance and exhibit high MYC and BMI1 activity.127
MPN myelofibrosis (MF) inducing fibrocytes were shown to upregulate GLI1 expression in a JAK2 and STAT3 signaling-dependent manner, thereby activating profibrotic signals required for remodeling of the microenvironment, for example, MMP2, MMP9, COL1A, and TGF-β signaling.128 In addition, the stromal compartment of the GLI1+ fibrotic MPN niche129 was remodeled in a SMO-mediated manner, giving rise to, for example, MMP2-, MMP9-, BMP2-expressing and TLR4- and NF-κB–activated cancer-associated fibroblasts.130,Gli1 inactivation ameliorates MF and antagonizes BM failure.129 Notably, similar to the loss of Ptch2 within MPN cells, loss of Ptch2 in niche cells increased disease aggressiveness in a JAK2V617F-driven MPN mouse model.117 Although not functionally analyzed, stromal cells isolated from mice mimicking chronic phase CML express reduced Ptch1 but not Gli1 levels.123 Also, in MDS and AML, the stromal compartment was modified by the tumor cells, resulting in methylation of the HHIP promoter, decreased expression of HHIP, and consequently improved leukemic cell support through HH ligand-induced signaling. Importantly, azacitidine treatment restored HHIP expression to normal levels and reduced leukemic cell support.102
Disrupting HH/GLI signaling to target survival and chemoresistance
Drug resistance is a major challenge in oncology, often caused by increased survival and decreased drug sensitivity of tumor cells. Combined targeting of antiapoptotic molecules such as BCL2 and prosurvival pathways, including HH/GLI, which directly controls BCL2 expression,131,132 offers promising therapeutic approaches to ameliorate chemoresistance.
Several studies have investigated the effect of HH inhibition and chemotherapeutics on MM cell viability. Both SMO and GLI targeting resulted in dose- and time-dependent apoptosis of MM cells, which correlated with decreased expression of prosurvival (BCL-2, SURVIVIN) and NOTCH signaling proteins, but increased expression and activity of proapoptotic factors (BAX, caspase 3).66,69 When analyzing MM cell resistance to bortezomib, increased GLI2 stability and activity due to abrogated DKK1-induced WWP2 E3 ligase activity and SIRT1 deacetylase overexpression were critical for MM cell survival.133,134 Consistent with these observations, the histone deacetylase inhibitor trichostatin A reduced GLI1 expression, resulting in downregulation of SURVIVIN and increased poly(ADP-ribose) polymerase (PARP) cleavage.135 Furthermore, autocrine SHH signaling counteracted spontaneous and drug-induced MM cell apoptosis,72 and stromal SHH signaling resulted in TRAF6 ubiquitination, canonical NF-κB signaling, and increased chemoresistance. Combined treatment with SMOi and bortezomib effectively killed MM cells, suggesting that administration of HH inhibitors may improve existing MM treatments.66,136
Comparable modes of action have been described for lymphomas, where upregulation of HH signaling occurs during the development of chemoresistance.137 SMO inhibition in NKTCL cells reduced BCL2 levels and induced apoptosis.86,138 Abrogation of HH signaling resulted in reduced STAT3 activity and BCL2 expression and coincident apoptosis of T-cell lymphoma cell lines.74,75,139 Similarly, Burkitt lymphoma and CLL cells showed reduced viability after GANT61 treatment, which was associated with autophagy induction in lymphoma cells and apoptosis in CLL, which was further increased by AKT inhibition.84,85,140-142 In CLL, only 37% of all patient samples tested responded to SMOi, representing mainly trisomy 12 CLL cases with high GLI1 and PTCH1 expression and autocrine DHH signaling. However, resistance to HH inhibition was overcome by additional blockade of DHH or ERK signaling or by depriving CLL cells of stromal support.142-144 Similarly, in MYC-driven murine B-cell lymphoma cells, SMOi treatment resulted in apoptosis that was counteracted by coculture with HH ligand-secreting stromal cells or overexpression of HH-promoting FUSED or GLI1.65 In addition, downregulation of either GLI1 or GLI2 reduced BCL2 expression and enhanced the antitumoral effect of doxorubicin.78 Consistent with the observation that SMO, but not GLI, activates NF-κB signaling in a PKC dependent manner, combined targeting of HH and NF-κB signaling rapidly reduced BCL2 and BCL-xL mRNA levels and induced apoptosis of DLBCL cells.145 Similarly, a close link between HH and AKT signaling has been established in DLBCL, as SMO or GLI1 inhibition resulted in decreased AKT1/2/3 transcription and cell survival.146 Notably, SMO inhibition alone predominantly induced apoptosis in ABC-type DLBCL cell lines because of decreased BCL2 and increased BAX expression, and combined inhibition of BCL2 and SMO overcame the resistance of GC-type DLBCL cell lines to apoptosis.88,147
HH pathway mutations also predict chemoresistance in individuals with T-ALL. In Jurkat cells harboring a PTCH1 mutation, it was shown that re-expression of wild-type PTCH1 led to downregulation of GLI1 mRNA, increased caspase 3/7 activity, and decreased cell viability, whereas chemical SMO activation by Smoothened agonist (SAG) counteracted these effects.92 Consistent with these observations, SMOi treatment resulted in increased death of T-ALL CEM and patient-derived ALL cells. Interestingly, HH pathway activity was regulated in a PKA-dependent manner and correlated with glucocorticoid resistance in CEM cells,148 thereby suggesting a cross talk between GLI and glucocorticoid receptor signaling. This was confirmed in cotreatment studies combining GANT61 and dexamethasone, which showed increased apoptosis of T-ALL cells in vitro and in vivo.149 Furthermore, combination therapies involving the addition of GANT61 or vismodegib to cytarabine or doxorubicin showed reduced chemoresistance in T-ALL cells with active HH signaling.91
Reflecting their differential requirement for SMO-dependent and -independent HH/GLI signaling, MUTZ1 MDS but not MDS-L cells underwent apoptosis upon inhibition of SMO, whereas GLI inhibition affected both cell lines.95-97 In addition, coculture of MUTZ1 cells with BM stromal cells from patients with high-risk MDS activated HH/GLI signaling and increased the expression of DNMT1, which was reversed by treatment with SMOi. Consistent with these observations, inhibition of DNA methylation using azacitidine in combination with cyclopamine resulted in significantly increased apoptosis in MDS cells.97,99
In AML, GLI1 overexpression can promote leukemic cell viability and chemoresistance through the upregulation of cell cycle regulators such as cyclin D and CDKs, as well as PI3K/AKT signaling. SMO inhibition resulted in reduced AML cell viability, downregulation of the PI3K/AKT/NF-κB and DNA repair pathways, and resensitization of leukemic cells to irradiation. Combined targeting of GLI1, FLT3, and PI3K resulted in increased AML cell apoptosis. In addition to inducing AML cell death, GLI1 and CDK4/6 inhibition also synergistically increased cytarabine sensitivity of AML cells.101,107,110,112,121,150 The downregulation of GLI3 in AML samples correlated with the upregulation of the chemoresistance-inducing genes SAMHD1, CDA, and MRP8 and cytarabine resistance.151 Importantly, these findings were confirmed in vivo using transgenic AML mouse models and xenografts.110,111,121 (for further details, see Tesanovic et al108)
In contrast to BCR-ABL− MPNs, several studies have established an association between HH signaling, elevated expression of antiapoptotic molecules, increased tumor cell survival, and LSC chemoresistance to ABL kinase inhibitors in BCR-ABL+ CML42,113,116,122,123,152,153 that is mediated, at least in part, by the tumor microenvironment.154 SMOi treatment downregulated AKT/mTOR signaling, induced autophagy, and negatively affected the viability of both imatinib-sensitive and -resistant CML BM cells and cell lines.42,113,114,122,155 Also, dual inhibition of SMO signaling and autophagy resensitized resistant CML cells to imatinib without altering the viability of healthy PBMCs.155 HH-regulated drug resistance is further supported by upregulation of the HH signaling core components in adriamycin-resistant K562 cells and by resensitization of leukemic cells to adriamycin by genetic GLI1 inhibition.156 In addition, SMO inhibition sensitized LSCs to and increased the potency of second-generation tyrosine kinase inhibitors, which significantly reduced the number of functional LSCs.42,113,115,116
Hopes and fails: HH/GLI targeting in clinical trials
Although preclinical in vitro and in vivo studies strongly support the idea of inhibiting HH/GLI signaling as a strategy to target malignant hematopoietic cells, clinical trials of established HH inhibitors have yielded mixed results. So far, clinical trials have exclusively evaluated SMOis, leaving room for future studies with inhibitors specific for GLI or its transcriptional cofactors, for example, BET bromodomain proteins.22,157 Since the FDA-approval of vismodegib in 2012,158 several other SMOis have been or are being tested in clinical trials for various types of cancer, including hematologic malignancies (Table 1). Although most SMOis are relatively well-tolerated with manageable adverse events, it has become clear that monotherapeutic approaches are rather ineffective in targeting the tumor (driving) cell population, irrespective of the hematologic disease. However, several studies have highlighted the potential of SMOis in combination therapy. Among these studies, the phase 2 Bright AML 1003 trial (NCT01546038) stands out, showing that the addition of glasdegib to low-dose cytarabine improved overall survival (8.8 vs 4.9 months) and complete remission rates (19.2% vs 2.6%) in AML and patients with high-risk MDS ineligible for intensive chemotherapy. The clinical benefit of glasdegib with a low-dose cytarabine regimen was particularly pronounced in patients with secondary AML, leading to FDA-approval of the treatment for high-risk AML.159-164 Despite its efficacy, glasdegib has not been widely used in the clinic due to the success of low-dose cytarabine or azacitidine in combination with the BCL2 inhibitor venetoclax. However, more selective, and personalized regimens can be envisioned and are partially included in ongoing phase 2 and 3 clinical trials. These combine glasdegib with drugs targeting mechanisms induced by recurrent AML mutations, such as HMAs, proapoptotic agents, FLT3 kinase inhibitors, and isocitrate dehydrogenase (IDH) inhibitors (NCT04842604, NCT03226418, NCT04168502, NCT04093505). Beyond AML and MDS, success stories for SMOis are rare but nevertheless encouraging. In CML, BMS-833923 resensitized patients to dasatinib (NCT01218477).165 In MF, combining sonidegib but not vismodegib with the JAK inhibitor ruxolitinib improved patient outcomes (NCT01787552, NCT02593760)166,167 and in MM, the combination of sonidegib and lenalidomide resulted in improved CR and response rates after stem cell transplantation, highlighting its potential as maintenance therapy (NCT02086552).168
Clinical trials of SMOis in hematopoietic malignancies
| Inhibitor . | Disease . | Dosage . | Combination . | (Preliminary) results . | Clinical trial . | Phase . | Status . | Reference(s) . |
|---|---|---|---|---|---|---|---|---|
| BMS-833923 | CML | 50-200 mg/d | Dasatinib | 30% of patients who are dasatinib-resistant with chronic phase CML responded to combination treatment (1 with complete cytogenetic response after 47-51 wk) | NCT01218477 | 1/2 | Completed | 165 |
| Leukemia | Not defined | Dasatinib | — | NCT01357655 | 2 | Terminated | — | |
| Glasdegib | AML | 100 mg/d | Cytarabine/daunorubicin, azacitidine | No improvement of OS through addition of glasdegib | NCT03416179 | 3 | Completed | 170 |
| AML | Not defined | SCT + bosutinib, decitabine, enasidenib, ivosidenib, venetoclax, gilteritinib | — | NCT04655391 | Pilot/1b | Withdrawn | — | |
| AML | 100 mg/d | Gemtuzumab ozogamicin or SCT | — | NCT04168502 | 3 | Recruiting | — | |
| AML | 100 mg/d | Decitabine | — | NCT04051996 | 2 | Terminated | — | |
| AML | 100 mg/d | Gemtuzumab ozogamicin | — | NCT04093505 | 3 | Recruiting | 171 | |
| AML | 100 mg/d | Gemtuzumab ozogamicin | 1 of 3 patients with reduced BM blasts after 1 cycle, no formal response | NCT03390296 | 1b/2 | Active | 172 | |
| AML | 100 mg/d | Decitabine, azacitidine, venetoclax | — | NCT03226418 | 2 | Active, not recruiting | 173 | |
| AML | Not defined | — | — | NCT04230564 | — | Withdrawn | — | |
| AML, CML, CMML, MDS, MF | 5, 10, 20, 40, 80, 120, 180, 270, 400, or 600 mg/d | — | Established MTD: 400 mg/d; AEs within the expected range; 2 patients experienced DLT (80 and 600 mg/d); clinical activity in 49% of patients | NCT00953758 | 1 | Completed | 174,175 | |
| AML, CMML, MDS | 50, 75, or 100 mg/d | Azacitidine | — | NCT04842604 (continuation of NCT03416179 and NCT02367456) | 3 | Active | — | |
| AML, MDS | 25, 50, or 100 mg/d | LDAC, azacitidine, cytarabine/daunorubicin | Glasdegib + LDAC, well-tolerated; glasdegib + azacitidine, well-tolerated; glasdegib + cytarabine/daunorubicin, manageable despite increased severe AEs; low remission rates in glasdegib only cohort, increased in combination cohorts | NCT02038777 | 1 | Active, not recruiting | 176 | |
| AML, MDS | 1b: 100 or 200 mg/d, 2: 100 mg/d | LDAC, decitabine, cytarabine/daunorubicin | Phase 1b: glasdegib in combination with LDAC or decitabine, well-tolerated; glasdegib + cytarabine/daunorubicin, increased severe AEs, manageable; 31% remission rate across all groups; phase 2: glasdegib + cytarabine/daunorubicin, high CR rate; glasdegib + LDAC, drastically improved CR rate and OS in combination compared to LDAC only (17% vs 2.3% and 8.8 vs 4.9 mo) | NCT01546038 | 1b/2 | Completed | 159-164 | |
| AML, MDS | 100 mg/d | Azacitidine | Well-tolerated; 20% of patients with AML and 13.3% of patients with MDS achieved CR | NCT02367456 | 1b | Completed | 177 | |
| AML, MDS | 100 mg/d | CPX-351 (cytarabine/daunorubicin) | — | NCT04231851 | 2 | Recruiting | — | |
| AML, MDS | 100 mg/d | SCT | 52% severe AEs, limited ability for glasdegib to prevent relapse in high-risk, post-SCT setting | NCT01841333 | 2 | Completed | 178 | |
| CML | 200, 400, or 600 mg/d | Nilotinib | Observed SAEs consistent with sonidegib safety profile, no evidence of clinical benefit for the combination for phase 2 trials | NCT01456676 | 1 | Completed | — | |
| CMML, MDS | 100 mg/d | — | 31% severe AEs, 54% stable disease, 6.4 mo progression-free survival | NCT01842646 | 2 | Completed | — | |
| MF | 100 mg/d | — | 24% severe AEs, no significant spleen volume reduction | NCT02226172 | 1/2 | Terminated | 179 | |
| Saridegib | MF | 60, 130, or 110 mg/d | — | 85% slight spleen size reduction, reduced fibrosis and JAK2V617F allele burden in some patients, too little of a response to further evaluate saridegib as a monotherapy | NCT01371617 | 2 | Completed | 180 |
| Sonidegib | ALL, AML | 2 × 400 or 800 mg/d | — | 100% of patients experienced ≥1 AE, 71% of patients experienced serious AEs; low CR rate (1.45% of patients), disease progression in 62% of patients | NCT01826214 | 2 | Completed | — |
| MF | 400 mg/d | Ruxolitinib | Well-tolerated; 44% reduction in spleen volume, ruxolitinib and sonidegib combination might provide improved benefit over ruxolitinib monotherapy | NCT01787552 | 1, 2 | Completed | 166 | |
| MM | 400 or 800 mg/d | Bortezomib | Safety lead-in data did not support continuation of study | NCT02254551 | 2 | Terminated | — | |
| MM | 400 mg/d | SCT, lenalidomide | 18% serious AEs; 46% complete response, 73% 2-y progression free survival rate | NCT02086552 | 2 | Completed | 168 | |
| Myeloid malignancies | 400 mg/d | Azacitidine | AEs within the expected range; remission rates comparable to azacitidine monotherapy, promising progression-free and overall survival | NCT02129101 | 1/1b | Completed | 181,182 | |
| Ptch1 or SMO mutated hematologic malignancies (except ALL, AML, CML) | 800 mg/d | — | 0% CR rate, 0% 16-week progression-free survival rate | NCT02002689 | 2 | Terminated | — | |
| Vismodegib | AML | 150 mg/d | Ribavirin, decitabine | Well-tolerated; shortened time to response, partial remissions, blast responses, and stable diseases | NCT02073838 | 2 | Completed | 169 |
| AML, MDS | 150 mg/d | (Cytarabine) | Vismodegib well-tolerated; lower-than-expected efficacy for vismodegib monotherapy; no combination therapy performed | NCT01880437 | 1b/2 | Terminated | 183 | |
| CLL, NHL | 150 mg/d | — | 52% of patients experienced ≥1 AE, 13% of patients experienced serious AEs; rapid disease progression in 90% of patients | NCT01944943 | 2 | Terminated | 184 | |
| MF | 150 mg/d | Ruxolitinib | Vismodegib well-tolerated; no improvement compared to ruxolitinib monotherapy | NCT02593760 | 1 | Completed | 167 | |
| MM | Not defined | SCT | — | NCT01330173 | 1 | Completed | — | |
| Various | Not defined | Not defined | — | NCT03878524 | 1b | Recruiting | — |
| Inhibitor . | Disease . | Dosage . | Combination . | (Preliminary) results . | Clinical trial . | Phase . | Status . | Reference(s) . |
|---|---|---|---|---|---|---|---|---|
| BMS-833923 | CML | 50-200 mg/d | Dasatinib | 30% of patients who are dasatinib-resistant with chronic phase CML responded to combination treatment (1 with complete cytogenetic response after 47-51 wk) | NCT01218477 | 1/2 | Completed | 165 |
| Leukemia | Not defined | Dasatinib | — | NCT01357655 | 2 | Terminated | — | |
| Glasdegib | AML | 100 mg/d | Cytarabine/daunorubicin, azacitidine | No improvement of OS through addition of glasdegib | NCT03416179 | 3 | Completed | 170 |
| AML | Not defined | SCT + bosutinib, decitabine, enasidenib, ivosidenib, venetoclax, gilteritinib | — | NCT04655391 | Pilot/1b | Withdrawn | — | |
| AML | 100 mg/d | Gemtuzumab ozogamicin or SCT | — | NCT04168502 | 3 | Recruiting | — | |
| AML | 100 mg/d | Decitabine | — | NCT04051996 | 2 | Terminated | — | |
| AML | 100 mg/d | Gemtuzumab ozogamicin | — | NCT04093505 | 3 | Recruiting | 171 | |
| AML | 100 mg/d | Gemtuzumab ozogamicin | 1 of 3 patients with reduced BM blasts after 1 cycle, no formal response | NCT03390296 | 1b/2 | Active | 172 | |
| AML | 100 mg/d | Decitabine, azacitidine, venetoclax | — | NCT03226418 | 2 | Active, not recruiting | 173 | |
| AML | Not defined | — | — | NCT04230564 | — | Withdrawn | — | |
| AML, CML, CMML, MDS, MF | 5, 10, 20, 40, 80, 120, 180, 270, 400, or 600 mg/d | — | Established MTD: 400 mg/d; AEs within the expected range; 2 patients experienced DLT (80 and 600 mg/d); clinical activity in 49% of patients | NCT00953758 | 1 | Completed | 174,175 | |
| AML, CMML, MDS | 50, 75, or 100 mg/d | Azacitidine | — | NCT04842604 (continuation of NCT03416179 and NCT02367456) | 3 | Active | — | |
| AML, MDS | 25, 50, or 100 mg/d | LDAC, azacitidine, cytarabine/daunorubicin | Glasdegib + LDAC, well-tolerated; glasdegib + azacitidine, well-tolerated; glasdegib + cytarabine/daunorubicin, manageable despite increased severe AEs; low remission rates in glasdegib only cohort, increased in combination cohorts | NCT02038777 | 1 | Active, not recruiting | 176 | |
| AML, MDS | 1b: 100 or 200 mg/d, 2: 100 mg/d | LDAC, decitabine, cytarabine/daunorubicin | Phase 1b: glasdegib in combination with LDAC or decitabine, well-tolerated; glasdegib + cytarabine/daunorubicin, increased severe AEs, manageable; 31% remission rate across all groups; phase 2: glasdegib + cytarabine/daunorubicin, high CR rate; glasdegib + LDAC, drastically improved CR rate and OS in combination compared to LDAC only (17% vs 2.3% and 8.8 vs 4.9 mo) | NCT01546038 | 1b/2 | Completed | 159-164 | |
| AML, MDS | 100 mg/d | Azacitidine | Well-tolerated; 20% of patients with AML and 13.3% of patients with MDS achieved CR | NCT02367456 | 1b | Completed | 177 | |
| AML, MDS | 100 mg/d | CPX-351 (cytarabine/daunorubicin) | — | NCT04231851 | 2 | Recruiting | — | |
| AML, MDS | 100 mg/d | SCT | 52% severe AEs, limited ability for glasdegib to prevent relapse in high-risk, post-SCT setting | NCT01841333 | 2 | Completed | 178 | |
| CML | 200, 400, or 600 mg/d | Nilotinib | Observed SAEs consistent with sonidegib safety profile, no evidence of clinical benefit for the combination for phase 2 trials | NCT01456676 | 1 | Completed | — | |
| CMML, MDS | 100 mg/d | — | 31% severe AEs, 54% stable disease, 6.4 mo progression-free survival | NCT01842646 | 2 | Completed | — | |
| MF | 100 mg/d | — | 24% severe AEs, no significant spleen volume reduction | NCT02226172 | 1/2 | Terminated | 179 | |
| Saridegib | MF | 60, 130, or 110 mg/d | — | 85% slight spleen size reduction, reduced fibrosis and JAK2V617F allele burden in some patients, too little of a response to further evaluate saridegib as a monotherapy | NCT01371617 | 2 | Completed | 180 |
| Sonidegib | ALL, AML | 2 × 400 or 800 mg/d | — | 100% of patients experienced ≥1 AE, 71% of patients experienced serious AEs; low CR rate (1.45% of patients), disease progression in 62% of patients | NCT01826214 | 2 | Completed | — |
| MF | 400 mg/d | Ruxolitinib | Well-tolerated; 44% reduction in spleen volume, ruxolitinib and sonidegib combination might provide improved benefit over ruxolitinib monotherapy | NCT01787552 | 1, 2 | Completed | 166 | |
| MM | 400 or 800 mg/d | Bortezomib | Safety lead-in data did not support continuation of study | NCT02254551 | 2 | Terminated | — | |
| MM | 400 mg/d | SCT, lenalidomide | 18% serious AEs; 46% complete response, 73% 2-y progression free survival rate | NCT02086552 | 2 | Completed | 168 | |
| Myeloid malignancies | 400 mg/d | Azacitidine | AEs within the expected range; remission rates comparable to azacitidine monotherapy, promising progression-free and overall survival | NCT02129101 | 1/1b | Completed | 181,182 | |
| Ptch1 or SMO mutated hematologic malignancies (except ALL, AML, CML) | 800 mg/d | — | 0% CR rate, 0% 16-week progression-free survival rate | NCT02002689 | 2 | Terminated | — | |
| Vismodegib | AML | 150 mg/d | Ribavirin, decitabine | Well-tolerated; shortened time to response, partial remissions, blast responses, and stable diseases | NCT02073838 | 2 | Completed | 169 |
| AML, MDS | 150 mg/d | (Cytarabine) | Vismodegib well-tolerated; lower-than-expected efficacy for vismodegib monotherapy; no combination therapy performed | NCT01880437 | 1b/2 | Terminated | 183 | |
| CLL, NHL | 150 mg/d | — | 52% of patients experienced ≥1 AE, 13% of patients experienced serious AEs; rapid disease progression in 90% of patients | NCT01944943 | 2 | Terminated | 184 | |
| MF | 150 mg/d | Ruxolitinib | Vismodegib well-tolerated; no improvement compared to ruxolitinib monotherapy | NCT02593760 | 1 | Completed | 167 | |
| MM | Not defined | SCT | — | NCT01330173 | 1 | Completed | — | |
| Various | Not defined | Not defined | — | NCT03878524 | 1b | Recruiting | — |
AE, adverse event; CR, complete remission; DLT, dose-limiting toxicity; LDAC, low-dose cytarabine; MTD, maximum tolerated dose; OS, overall survival; SAE, severe adverse event; SCT, stem cell transplantation.
Given the heterogeneity observed among patients, personalized treatment regimens tailored to the patient's tumor promoting genetic makeup and pathway activities will be a critical aspect of treating hematologic malignancies with prolonged success or even curative outcomes. As recently demonstrated, one potential target in this context is HH/GLI-mediated resistance to ribavirin and cytarabine. In relapsed patients with AML with GLI-driven expression of UGT1A, responsible for drug glucuronidation and inactivation, vismodegib downregulated UGT1A expression and resensitized AML cells to ribavirin and decitabine, resulting in shortened time to response, partial remissions, blast responses, and stable disease. Importantly, 3 of 5 patients with progressive disease harbored FLT3-ITD mutations, which correlate with SMO-independent GLI signaling.169 Therefore, future clinical trials should assess HH/GLI activity within tumor cells and the tumor-supporting microenvironment of patients before treatment. This should include examining HH ligand responsiveness and the presence of cilia-like structures as possible predictive biomarkers of active SMO-dependent HH signaling. The combination of SMO/GLI inhibitors with other treatment modalities, such as HMAs, proapoptotic agents, IDH inhibitors, tyrosine kinase inhibitors, protease inhibitors, pyrimidine analogs, immunotherapies, and senolytics holds great promise for tackling hard-to-treat patient subgroups. In this context, combining HH inhibitors with the BCL2 inhibitor venetoclax appears to be an intriguing and feasible approach to first block the cancer cells' hallmark abilities, to sensitize them to further treatments, and ultimately to eliminate them.
Acknowledgments
The authors are grateful for critical discussions with members of the Cancer Cluster Salzburg and apologize to all colleagues whose work is not cited due to space limitations. Figures were generated with Biorender.com.
This work was supported by the following grants: Austrian Science Fund (FWF) project W1213, the EU Interreg Project EPIC (ITAT 1054), the Cancer Cluster Salzburg projects 20102-P1601064-FPR01-2017 and 20102-F2001080FPR, the Biomed Center Salzburg project 20102-F1901165KZP, and the priority program “Allergy-Cancer-Bionano Research Center” of the University of Salzburg.
Authorship
Contribution: P.W.K. and F.A. conceived the content and structure of the review, wrote the manuscript, and designed the figures.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter W. Krenn, Department of Biosciences and Medical Biology, Cancer Cluster Salzburg, Paris Lodron University of Salzburg, Hellbrunner Strasse 34, 5020 Salzburg, Austria; e-mail: peter.krenn@plus.ac.at; and Fritz Aberger, Department of Biosciences and Medical Biology, Cancer Cluster Salzburg, Paris Lodron University of Salzburg, Hellbrunner Strasse 34, 5020 Salzburg, Austria; e-mail: fritz.aberger@plus.ac.at.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal