Introduction
Megakaryocytes (MKs) have familiar roles in the bone marrow (BM) microenvironment, including platelet production, maintenance of hematopoietic stem and progenitor cells (HSPCs) function, and more recent evidence suggests MK engagement in immune responses. Immune aplastic anemia (AA) is characterized by oligoclonal expansion of T-cells and death of HSPCs leading to bone marrow failure (BMF). As MKs demonstrate niche maintenance and immune effector function, we explored the potential roles of MKs in the development of AA in a murine model of immune BMF.
Methods
BMF was induced in CByB6F1 mice by intravenous infusion of lymph node (LN) cells from major histocompatibility complex mismatched C57BL/B6 (B6) donor mice at a dose of 30-40x10 6 cells/recipient. No irradiation was given to recipients to avoid extra damage to MKs and CByB6F1 recipient mice were sacrificed at D7, D10, D14, and D17 following LN cell infusion (Chen J, et al. Blood. 2004). Peripheral blood (PB) samples, tibias, femurs, and sterna were harvested at each timepoint. Immunophenotyping of MKs and T cells was performed by flow cytometry on flushed BM cells and two-photon excitation microscopy (2P) imaging was performed on fixed sternal samples, using fluorescence-labelled CD41 antibody to visualize the physical location of MKs in BM. To enrich for MKs, BM cells were filtered through a 10 µm pluriStrainer™ through which most lymphocytes, red blood cells, and other small cells were eluted while MKs and other large size cells remained on the strainer. Large cells and residual small cells were subsequently assessed by single cell RNA sequencing (sc-RNAseq) on the Parse Bio Evercode™ platform based on combinatorial barcoding technology, which was able to accommodate the large size of MKs, as no partitioning step is needed in the analysis workflow.
Results
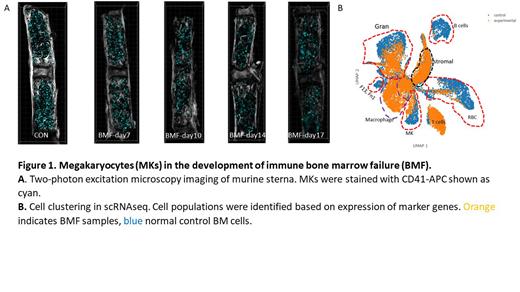
BMF was successfully induced as measured by decreasing PB counts from point of lymphocyte infusion accompanied by increasing expansion of CD4 and CD8 T-cells and marked reduction of total BM cells. MKs persisted throughout the course of BMF as measured by both flow cytometry and 2P (Figure 1A) despite PB thrombocytopenia and progressive marrow hypocellularity. Concomitantly, MKs identified by large size on forward scatter versus side scatter and CD41+ expression had progressive upregulation of immune effector markers IA-IE and CD53 over the course of BMF. Following sc-RNAseq and bioinformatics analysis of enriched BM cells at D14, cell clustering identified a distinct cell cluster representing MKs (Figure 1B). MK identity was supported by co-expression of transcripts encoding platelet factor 4, TUBB1, Glycoprotein 5, and Itga2b (CD41). This MK cell population was distinct from macrophages which highly expressed Fcgr4, Cd5l, Ccr5, Cd74 and Gbp5 genes. Gene expression profiling demonstrated that MKs in BMF mice showed upregulation of immune pathways such as interferon gamma and interferon alpha response and downregulation of pathways related to platelet activation and platelet homeostasis, as compared with normal mice.
Conclusions
In a murine model of BMF, using multiple modalities, we demonstrate that MKs cease platelet production, manifested by reduction in peripheral blood platelet count and downregulation of gene expression pathways related to platelet production, while concurrently acquiring characteristics of immune effector cells. This suggests that MKs are active participants in immune BMF.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal