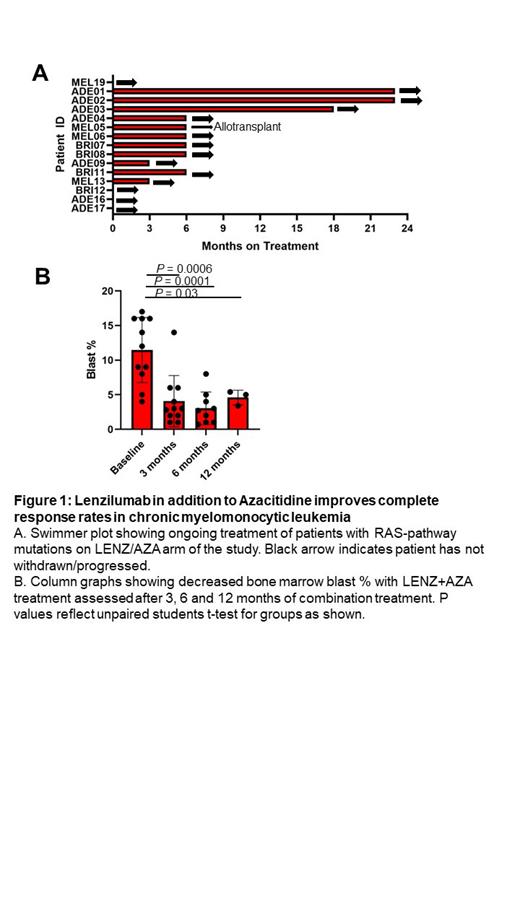
Introduction: Chronic myelomonocytic leukemia (CMML) is a rare cancer orchestrated by granulocyte-macrophage colony-stimulating factor (GM-CSF), a pro-inflammatory cytokine that drives leukemic monocyte proliferation. Standard of care (SOC) for CMML treatment includes azacitidine (AZA), with a complete response (CR) rate of 16-21% 1,2. The PREcision Approach to CHronic Myelomonocytic Leukemia (PREACH-M; ACTRN12621000223831) trial investigates novel CMML therapies directed by molecular profiling. Lenzilumab (LENZ; Humanigen, Inc., Short Hills, NJ) a proprietary Humaneered® first-in-class monoclonal antibody with best-in-class off-rate and affinity that neutralizes GM-CSF. PREACH-M interim results show that LENZ/AZA improves hematologic parameters, decreases spleen size, and dampens pro-inflammatory responses in CMML with RAS-pathway mutations. This report details the objective clinical responses from an interim analysis of the first 11 subjects who completed at least three months LENZ/AZA treatment. Methods: PREACH-M is a Phase 2/3 non-randomized, uncontrolled, open-label trial in 72 adults aged at least 18 years, newly diagnosed with WHO 2016 criteria for CMML. Key exclusion criteria include prior treatment with investigational agents; radiotherapy within 28 days before treatment; treatment with G-CSF within 7 days of screening; GM-CSF within 28 days of screening; and uncontrolled medical conditions. Subjects exhibiting RAS-pathway mutations ( NRAS, KRAS, CBL) receive 24 cycles (every 28 days) of AZA (SC; 75 mg/m2 for 7 days) and LENZ (IV; 552 mg; d1 & d15 of cycle 1 and d1 only for all subsequent cycles); while those with only TET2 mutations receive the same AZA regimen and sodium ascorbate (IV; 30 g for 7 days [15 g for 1st dose only, 30 g thereafter if no evidence of tumor lysis syndrome]; PO; 1.1g on all other days). Subjects who complete 24 cycles of treatment are followed every 6 months for an additional 24 months. The primary endpoint is the frequency of complete response (CR) or partial response (PR) during the first 12 cycles according to Savona Criteria. Secondary endpoints include responses according to modified 2006 International Working Group criteria, 2 year overall survival, and symptom improvement. Results: As of July 2023, 15 subjects were enrolled in the LENZ/AZA arm (8 females, 7 males with mean age 69; mean white cell count 21x10 9/L, mean Hb 121 g/L; mean platelet count, 74x10 9/L, mean blast count, 10.1%). Mutations included; CBL (47% of subjects), NRAS (27%), KRAS (47%), NRAS and KRAS (13%), and TET2 (93%). Subjects exhibited CPSS-MOL scores, of intermediate risk 1 (n=1), intermediate risk 2-3 (n=8), and high risk 4-6 (n=6). All the 11 evaluable subjects at 3 months responded to LENZ/AZA. CR was achieved within 3 cycles in 55% of subjects. Six of the subjects demonstrated CR including 2 with a high risk CPSS-MOL profile and 8 achieved either CR or complete marrow response (blasts<5%) within 12 months. One subject had a platelet response, 1 subject each had PR and stable disease with blasts <5%. CMML progression was absent and 1 subject became eligible for allogeneic transplant. These findings exceed historical CR rates for hypomethylating agents (16%; 95%CI, 12-21% 1 and 21%;13-29% 2). Self-reported symptom scores from the standardized MPN-SAFFS improved from baseline (mean of 22 vs 12, P=0.06). Fifteen grade 3 and 9 grade 4 adverse events were reported of which 2 were “probably” ascribed to both LENZ/AZA and 7 were “possibly” ascribed to LENZ. No unexpected adverse events were observed. Conclusion: Interim analysis of the PREACH-M trial demonstrated that GM-CSF neutralization with LENZ/AZA, for the treatment of CMML with RAS-pathway mutations resulted in 55% CR, achieved early in treatment, durability up to 18 months, thus far, and no unexpected serious adverse events. These data suggest CMML is driven by a non-redundant cytokine that responds to immunotherapy.
1. Xu Y, Guo R, Miao M, Zhang G, Lan J, Jin J. Real-world data on efficacy and safety of azacitidine therapy in chronic myelomonocytic leukemia in China: results from a multicenter, retrospective study. Invest New Drugs 2022;40(5):1117-1124. DOI: 10.1007/s10637-022-01283-x.
2. Zheng X, Lv L, Li X, Jiang E. Efficacy and Safety of Hypomethylating Agents in Chronic Myelomonocytic Leukemia: A Single-Arm Meta-analysis. Glob Med Genet 2022;9(2):141-151. DOI: 10.1055/s-0042-1744157.
OffLabel Disclosure:
Ross:Imago BioSciences, Inc., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA: Research Funding; Keros: Consultancy; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees; Menarini: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Honoraria, Research Funding. Fong:Astellas: Honoraria; Pfizer: Honoraria, Speakers Bureau; Jazz: Honoraria; BeiGene: Honoraria; Otsuka: Honoraria; Servier: Honoraria, Speakers Bureau; Novartis: Honoraria; BMS: Honoraria; AbbVie: Honoraria, Speakers Bureau; Amgen: Honoraria. Yong:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Research Funding; Celgene: Research Funding. Yeung:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Hughes:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Terns Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Enliven: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Lenzilumab is a humanized monoclonal antibody that neutralises granulocyte-macrophage colony-stimulating-factor. The drug is being used to treat patients with high risk chronic myelomonocytic leukaemia.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal