Background: Patients (pts) with CLL have a high rate of comorbidities, including cardiovascular disease (CVD). Comorbid CVD is correlated with higher mortality in this pt population. Hypertension (HTN) is the most common concurrent CVD in pts with CLL and is associated with development of complications such as stroke and heart failure. The incidence of new or worsening HTN in treatment-naive CLL pts receiving placebo within the CLL12 trial was 8.3% over a 69.3-month observation period (Langerbeins et al. Hematol Oncol. 2023). New and/or worsening HTN has been observed in pts with CLL treated with Bruton tyrosine kinase inhibitor (BTKi) therapy. Studies have shown new or worsening HTN with ibrutinib (7−30%) and zanubrutinib (11−20%) (Christensen et al. Expert Rev Hematol. 2022). Acalabrutinib (acala), a selective, second-generation BTKi, demonstrated a lower rate of HTN (8.6%) vs ibrutinib (22.8%) in the head-to-head ELEVATE-RR study at a median follow-up of 40.9 months (Byrd et al. J Clin Oncol. 2021). Here, we performed a cumulative analysis of new or worsening HTN from the acala global development program and compared these data against real-world prevalence and incidence of HTN for pts with CLL prior to treatment initiation using claims data.
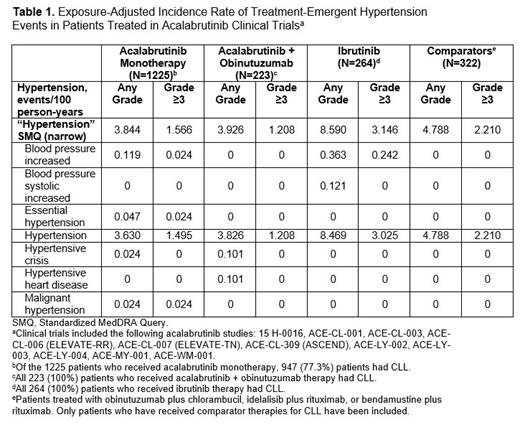
Methods: Safety reports of new or worsening HTN were obtained from 11 clinical trials with acala (the safety database analysis comprised 2034 pts from studies in CLL [86.3%] and mantle cell lymphoma [6.1%], with the remainder from other B-cell malignancies). The clinical trials analyzed included therapy with acala (monotherapy or in combination with obinutuzumab) and active comparator groups (described in Table 1). Using the narrow Standardized MedDRA Query and the related MedDRA preferred terms for “HTN,” exposure-adjusted incidence rates in events/100 person-years were determined, and as a comparison, HTN prevalence was assessed at median treatment exposure for acala monotherapy. HTN prevalence (proportion of pts with HTN 6 months before treatment initiation) and incidence rates (number of new cases of HTN per 100 person-years during the 5 years before any treatment initiation) were also assessed based on ICD-10 codes from 3 adjudicated closed claims databases (Optum, MarketScan, and IQVIA).
Results: In the claims database analysis, prevalence of HTN ranged from 47.3% to 66.1% in pts with TN CLL prior to treatment initiation. HTN prevalence with acala monotherapy at median treatment exposure (45.5 months) was 55.7%. Exposure-adjusted incidence rates of HTN were analyzed from 11 clinical trials; 1225 pts received acala monotherapy (947 pts with CLL), 223 pts received acala + obinutuzumab (all pts with CLL), 264 pts received ibrutinib as a comparator (all pts with CLL), and 322 pts received other comparators (all pts with CLL). Among the 1225 pts treated with acala monotherapy, 165 pts (147 with CLL) experienced a treatment-emergent adverse event of HTN (median age 66 years; 66.1% [n=109] male; 59.4% [n=98] with history of HTN at baseline). The exposure-adjusted incidence rates of HTN with acala monotherapy (3.844) and acala + obinutuzumab (3.926) were similar to that of other comparators (4.788 [excluding ibrutinib]) ( Table 1). In the ELEVATE-RR study specifically (subgroup of the current analysis), the exposure-adjusted incidence rate of HTN with acala (3.053) was approximately one-third that of ibrutinib (8.590). The exposure-adjusted incidence rate of HTN in pts treated with acala monotherapy without history of HTN was 3.239.
Conclusions: Our data showed that HTN is a common comorbidity in pts with CLL as demonstrated in the claims database and clinical trial database analyses where 59.4% of pts who experienced HTN with acala monotherapy had history of HTN at baseline. The incidence rates of new or worsening HTN with acala monotherapy and acala + obinutuzumab were low (3.844 and 3.926, respectively). This is supported by similar incidence proportion of new or worsening HTN in pts receiving placebo (8.3%) from CLL12 (median follow-up 69.3 months) and acala monotherapy (8.9%) from ELEVATE-TN (median follow-up 58.2 months; Sharman et al. EHA. 2022), and similar HTN prevalence at median treatment exposure vs before treatment initiation in claims databases. Our analysis, conducted predominantly in patients with CLL, suggests that acala monotherapy did not worsen pre-existing HTN or increase the risk of new-onset HTN.
Disclosures
Ferrajoli:Beigene: Research Funding; AstraZeneca: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; GenMab: Research Funding; Janssen: Honoraria; Genetech: Honoraria. Follows:Genesis Care: Consultancy, Honoraria; Centessa: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Beigene: Consultancy, Honoraria, Speakers Bureau; Lilly: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau. Marmor:AstraZeneca: Current Employment, Current holder of stock options in a privately-held company. Bourjlian:AstraZeneca: Current Employment. Roos:AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Bajwa:AstraZeneca: Current Employment. Madhira:AstraZeneca: Current Employment. Nozaki:AstraZeneca: Current Employment. Palhares De Miranda:AstraZeneca: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Patel:CRISPR Therapeutics: Research Funding; Pharmacyclics/Janssen: Consultancy, Research Funding; Sunesis Pharmaceuticals: Research Funding; Trillium Therapeutics/Pfizer: Consultancy, Research Funding; Xencor: Consultancy, Research Funding; Curis, Inc: Research Funding; Kite: Consultancy, Research Funding, Speakers Bureau; Caribou Biosciences: Consultancy; Nurix: Research Funding; MEI Pharma: Consultancy, Research Funding; TG Therapeutics: Consultancy, Speakers Bureau; ADC Therapeutics: Consultancy; Morphosys: Consultancy; Epizyme: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; Fate Therapeutics: Research Funding; Loxo Oncology: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding, Speakers Bureau; BeiGene: Consultancy; Merck: Consultancy, Research Funding; Adaptive Biotechnologies: Research Funding; Abbvie: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal