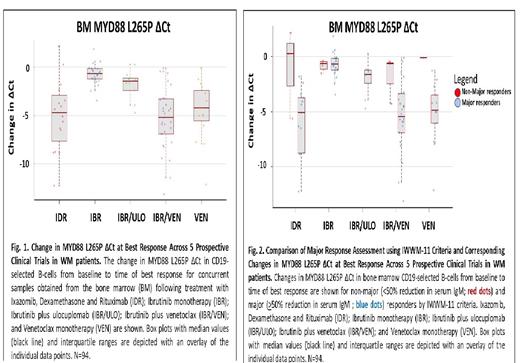
Serum IgM measurements represent the current standard for assessing changes in disease burden in Waldenström's Macroglobulinemia (WM). Many agents used to treat WM can significantly affect serum IgM levels without impacting the underlying burden of clonal lymphoplasmacytic cells thereby producing discordant findings. Such agents can raise (CD20-directed monoclonal antibodies, IMiDs), or lower (BTK-, proteasome-, MTOR- inhibitors) serum IgM levels, and impact categorical response assessment. Somatic mutations in MYD88 are found in 95-97% of WM patients, and support tumor growth through activation of multiple pro-survival pathways that include HCK/BTK, SYK and ERK. Nearly all MYD88 mutations in WM are of the L265P variant. Previous studies by us and others have shown that serial quantitative measurements of both bone marrow (BM) and peripheral blood (PB) MYD88 L265P (c.978 T>C) by allele-specific PCR can be used to assess changes in underlying BM disease burden (Blood 121:2051-2058; Leukemia 28:1698-1704). However, the utility of using quantitative allele-specific MYD88 L265P (qMYD88 L265P) analysis has not been studied in prospective clinical trials. As such, we performed a comprehensive study of qMYD88 L265P response assessment utilizing BM and PB CD19-selected tissue across 5 prospective clinical studies in WM: Ixazomib, Dexamethasone and Rituximab (IDR; NCT02400437) Ibrutinib monotherapy (IBR; NCT02604511); Venetoclax monotherapy (VEN; NCT02677324); Ibrutinib plus Ulocuplomab (IBR/ULO; NCT03225716); and Ibrutinib plus Venetoclax (IBR/VEN; NCT04273139). Changes in MYD88 L265P ΔCt were assessed as reported above with ΔCt = Mutant Ct - Wild-type Ct, and higher ΔCt values indicating a lower mutant allele burden, with each ΔCt unit reduction representing a 50% decrease in mutant MYD88 L265P burden. Changes in MYD88 L265P ΔCt were compared to changes in underlying BM disease burden and categorical response assessment using the current (IWWM-11) response criteria (Semin Hematol. 60:97-106). Across all five trials, BM (r=0.52; p<0.001) and PB (r=0.43; p<0.001) MYD88 L265P ΔCt changes from baseline were highly correlated at best response with corresponding changes in underlying BM disease burden determined by repeat BM biopsies. As shown in Fig. 1, comparing changes from baseline in BM MYD88 L265P ΔCt assessments across studies showed marked differences, with greatest reductions observed in patients treated with IBR/VEN, IDR, and VEN alone, than for those treated with IBR alone or IBR/ULO. Similar findings were also observed for corresponding PB MYD88 L265P ΔCt assessments from baseline, with changes in PB MYD88 L265P ΔCt strongly correlating with those of BM MYD88 L265P ΔCt findings across all 5 clinical trials (r=0.67; p<0.001), thereby signifying the ability to use PB MYD88 L265P ΔCt assessments to evaluate treatment responses. We next compared findings from BM and PB MYD88 L265P ΔCt to changes from baseline in serum IgM levels across all 5 trials. At best response, major categorical responses denoted by >50% reduction in serum IgM using IWWM-11 criteria showed commensurate decreases in BM and PB MYD88 L265P ΔCt from baseline in patients receiving IBR/VEN, IDR and VEN alone ( Fig. 2). In contrast, minimal changes in BM and PB MYD88 L265P ΔCt from baseline were observed at best response for most major responders on IBR or IBR/ULO, including individuals who achieved very good partial responses denoted by >90% decrease in IgM by IWWM-11 criteria. In this first prospective evaluation of BM and PB qMYD88 L265P response assessment, we show that both BM and PB L265P qMYD88 analysis can provide more accurate assessment of treatment related changes in disease burden over the current standard of IgM response assessment alone, and can be used to more robustly evaluate clinical trial performance byidentifying treatments or regimens that produce more meaningful tumor reductions in WM patients.
OffLabel Disclosure:
Branagan:Sanofi: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Genzyme: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; BeiGene: Membership on an entity's Board of Directors or advisory committees; Adaptive: Membership on an entity's Board of Directors or advisory committees. Sarosiek:Beigene: Honoraria, Research Funding; Cellectar: Consultancy, Research Funding; ADC Therapeutics: Research Funding. Castillo:Loxo: Consultancy, Research Funding; Cellectar: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Kite: Consultancy; Mustang Bio: Consultancy. Treon:Janssen: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Eli Lilly: Consultancy, Research Funding; Bristoll Myers Squibb: Consultancy, Research Funding; Abbvie/Pharmacyclics: Consultancy, Research Funding.
This is a biomarker study of multiple trials. Off label drug usage includes ixazomib, ulocuplomab, ventoclax

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal