Introduction
The risk of MDS among adolescents and young adults (AYA) with cancer treated with chemotherapy with or without radiotherapy in the US is not well understood.
Method
We interrogated NCI's Surveillance, Epidemiology and End Result (SEER)-17 registries to identify patients (pts) between the ages of 15-39 years (AYA) diagnosed with MDS at least one year after diagnosis of a primary unrelated cancer treated with chemotherapy and or radiotherapy over a twenty-year period (January 2000 to December 2020). All cancers, including MDS, were identified using ICD-O-3/WHO-2008 histology codes. We excluded all leukemia and MDS entities from index cancer sites. The risk of MDS was estimated by standardized incidence ratio (SIR), defined as the ratio of observed MDS cases in the AYA pts treated for first cancers to the expected number of MDS cases in the general US population during this period. A referent rate file for MDS incidence in age, gender, race and calendar-year matched US population available in SEER*Stat software (version 8.4.0) was used for SIR calculation. We calculated exact, 2-sided Poisson-based 95% confidence intervals (CIs) about the SIRs. We stratified the risk using 5- calendar year periods, and latency after index cancer diagnosis. SIR and time to MDS development was calculated for six most common index cancers using R(v4.2.2)-software.
Result
Of a total of 168,078 AYA pts eligible for analysis, with 1,290,803-person years at risk during 2000 to 2020, 66 pts developed MDS during this period. The median age at diagnosis of index cancer and MDS were 34 years (yrs) (interquartile range (IQR), 27-37 yrs) and 38 yrs (IQR, 32-44 yrs), respectively. There were 680% more cases of MDS in the AYA cohort compared to that expected in the matched general US population [SIR (95% CI): 7.80 (6.03-9.92), p<0.05)]. A higher SIR was observed for males (12.19) compared to females (5.82) and among all race/ethnicity, highest among black males [(SIR (95%CI): 18.99 (6.17-44.32), p<0.05].
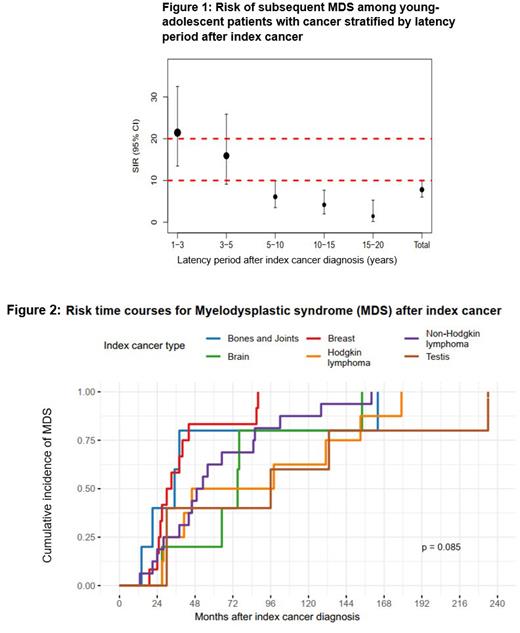
We analyzed SIR for top-six index cancer sites that contributed to most MDS cases. Lymphomas [Non-Hodgkin (NHL) and Hodgkin (HL)] and breast accounted for 54.5% of all MDS cases. SIR for MDS in pts with NHL was 22.07 (95% CI: 12.61-35.84), in HL pts was 10.75 (95% CI: 4.64-21.18), and in breast cancer pts was 2.79 (95% CI:2.22-7.51). Even though the absolute number of cases was low, pts with brain, as well as bone and joint cancers had highest SIRs for MDS. When stratified by 5-calendar year periods, the SIR of MDS was elevated compared to general population through all periods and was highest during 2000-2005 (27.44; 95% CI: 11.03-56.53), and declined for the subsequent time periods. When analyzed by latency period, the SIR for MDS was highest at 1-3 years after index cancer diagnosis (21.46: 95% CI 13.45-32.50) and then showed a steady decline with increasing latency over a period of 15-20 years but never reached the rates observed in general population (Figure 1). Among the six most common index sites, pts with bone and joint cancer had earliest time to occurrence of MDS, whereas the longest latency to developing MDS was observed in pts with testicular cancer (Figure 2).
Conclusion
AYA cancer cohort treated with chemotherapy or radiotherapy have nearly 8-fold higher risk of future development of MDS compared to the same age matched general US population. This risk is highest during the initial 1-3 years of diagnosis of index cancer, declines over time, but never reaches the rates in the general population. These findings warrant further investigation to understand the biology behind this observation and have potential treatment and surveillance implications.
Disclosures
Singh:Rigel: Other: Advisor or review panel participant. Advani:Beam: Honoraria; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kura: Honoraria; Servier: Research Funding; Macrogenics: Research Funding; Seattle Genetics: Research Funding; Glycomimetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Immunogen: Research Funding; Kite: Honoraria, Other: consulting, Research Funding; Incyte: Research Funding; OBI: Research Funding; Pfizer: Honoraria, Research Funding; Amgen: Honoraria, Other: advisory board, Research Funding; Taiho: Honoraria, Membership on an entity's Board of Directors or advisory committees; Nkarta: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Carraway:Novartis: Consultancy, Other: Travel, Accommodations, Expenses , Speakers Bureau; BMS: Consultancy, Research Funding, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Other: Travel, Accommodations, Expenses , Speakers Bureau; Stemline Therapeutics: Consultancy, Speakers Bureau; Genentech: Consultancy; Daiichi: Consultancy; Celgene: Research Funding; Agios: Consultancy, Speakers Bureau; AbbVie: Other; Astex Pharmaceuticals: Other; Syndax: Other: DSMB; Takeda: Other. Gerds:AbbVie, Bristol Myers Squibb, Constellation Pharmaceuticals, GlaxoSmithKline, Kartos, Novartis, PharmaEssentia, Sierra Oncology: Consultancy; Accurate Pharmaceuticals, Constellation Pharmaceuticals, CTI BioPharma, Imago BioSciences, Incyte Corporation, Kratos Pharmaceuticals: Research Funding. Mukherjee:Celgene/Acceleron: Other: Advisory Board; Bristol Myers Squibb: Other: Advisory Board; Novartis: Other: Advisory Board; Blueprint Medicines Corporation: Other: Advisory Board; Genentech and AbbVie: Other: Advisory Board; EUSA: Other: Advisory Board; Aplastic Anemia and MDS International Foundation: Honoraria; Celgene (now BMS): Honoraria; Bristol Myers Squibb: Honoraria; McGraw Hill Hematology Oncology Board Review: Honoraria; EUSA: Honoraria; BioPharm: Consultancy; Celgene (now BMS): Consultancy; Novartis: Consultancy; Bristol Myers Squibb: Consultancy; Celgene (now BMS): Research Funding; Novartis: Research Funding; Jazz Pharmaceuticals: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal