Introduction: Luspatercept is an erythroid maturation agent that promotes late-stage erythropoiesis in patients with LR-MDS. While it is known that luspatercept, a modified activin receptor ligand trap, binds to GDF11 and Activin B, the mechanism by which these ligands trigger a decrease in red blood cell (RBC) formation is unknown. Luspatercept was approved by the FDA following the phase 3 MEDALIST study, but biomarkers of response are currently unknown.
Results:In a large cohort of primary MDS CD34+ samples, GDF11 expression was significantly higher in MDS samples than in age-matched healthy controls (183 MDS samples vs 17 controls, P < 0.05). GDF11 was detected in primary MDS sera and was substantially elevated in the refractory anemia (RA) and RA with ring sideroblasts (RARS) MDS subgroups. Activin receptor 2B (ACVR2B), the receptor of GDF11, was also overexpressed in MDS samples, and its expression was confirmed by immunohistochemistry (IHC) in the bone marrows of primary MDS patients. GDF11/ACVR2B activates SMAD2, and we found that the overexpression of SMAD2 in CD34+ cells was associated with more severe anemia in MDS patients. RNA-seq and SMAD2 ChIP-seq analyses were then performed to determine the downstream effects of GDF11 in erythroid progenitors. Human CD34+ primary cells were utilized to generate BFU-E stage erythroid progenitors. We discovered that GDF11 promotes an abnormal erythroid gene expression program characterized by increased expression of pro-apoptotic and early erythroid differentiation-related genes. SMAD2 binding was detected in regulatory regions of early erythroid (including GATA1), proinflammatory, and apoptotic transcripts using ChIP-seq analysis with p-SMAD2. In a zebrafish model, administration of GDF11 caused a decrease in erythroid cells, which was rescued by treatment with luspatercept (Wobus et al. Leukemia 2021 35(10):2936-47). Luspatercept inhibited the phosphorylation and activation of SMAD2 in response to GDF11 stimulation in primary hematopoietic cells and leukemia cell lines. Importantly, luspatercept could reverse the growth-inhibiting effects of MDS-derived sera on primary hematopoietic progenitors. Finally, to identify biomarkers associated with clinical responses, we analyzed phase 3 MEDALIST study samples collected before and after treatment with lupatercept. RNA-seq analysis was performed on bone marrow samples from responders (R; n = 14) and non-responders (NR; n = 9) (Figure). Analysis of pre-treatment/screening bone marrow transcriptomes revealed no significant differences between R and NR in global gene expression. Analysis of splice isoforms between the two cohorts revealed strikingly greater differences. Most differential splicing events were observed in NR (702 in R vs 5232 in NR). The most prevalent splicing modifications were exon skipping events, and most functional pathways affected by differential exon usage were RNA splicing related.
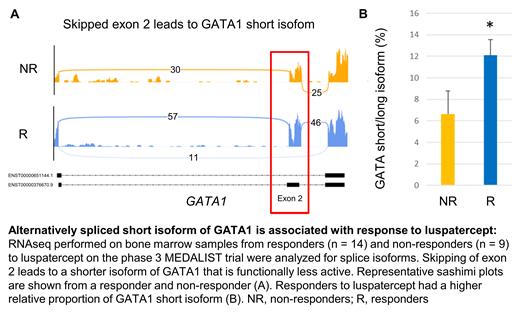
GATA1, the master erythroid transcription factor, was spliced differently in R at baseline, which was an intriguing observation. Response to luspatercept was found to be associated with exon 2 skipping, resulting in a shortened isoform of GATA1. R had a greater proportion of the short GATA1 isoform than NR (12% in R versus 6% in NR; P = 0.04). We analyzed ChIP-seq data from primary erythroid cells to determine the effect of GDF11/SMAD2 activation on GATA1 splicing. GDF11 stimulation resulted in SMAD2 binding to the second intron of GATA1. CRISPR-mediated deletion of the SMAD2 binding region in primary erythroid progenitors increased expression of the GATA1 long isoform. Next, we performed a CRISPR-mediated deletion of exon 2 of GATA1, resulting in a predominance of GATA1 short isoform expression and a decrease in erythroid output. In CRISPR-modified primary erythroid cells with a predominant short GATA1 isoform (exon 2-deleted cells), luspatercept was able to reverse the inhibition induced by GDF11.
Conclusions: These results demonstrate that MDS cases that respond to luspatercept are associated with a relatively high level of expression of functionally deficient GATA1 short isoform. We further demonstrate that GDF11 stimulates the production of the GATA1 short isoform via SMAD2 binding to intron 2 of the GATA1 gene. The regulation of a GATA1 splicing isoform by GDF11/SMAD2 is associated with clinical responses to luspatercept, according to these novel findings.
Disclosures
Fraint:Albert Einstein College of Medicine: Current Employment. Poigailwar:Albert Einstein College of Medicine: Current Employment. Zhao:Albert Einstein COM: Current Employment. Bachiashvili:University of Alabama at Birmingham: Current Employment. Steidl:Pfizer: Consultancy; Roche: Consultancy; Novartis: Consultancy; Vor Biopharma: Consultancy; Pieris Pharmaceuticals: Consultancy; Stelexis Therapeutics: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Aileron Therapeutics: Consultancy; Albert Einstein Medical College: Current Employment; Trillium Therapeutics: Consultancy. Wickrema:University of Chicago: Current Employment. Shastri:Gilead Sciences: Membership on an entity's Board of Directors or advisory committees; Kymera Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Rigel Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Vodala:Bristol Myers Squibb: Current Employment; Mabgenex: Membership on an entity's Board of Directors or advisory committees. Verma:Celgene: Consultancy; Prelude: Research Funding; Acceleron: Consultancy; Throws Exception: Current equity holder in private company; Medpacto: Research Funding; Curis: Research Funding; Eli Lilly: Research Funding; Janssen: Honoraria; Bakx: Consultancy, Current equity holder in private company; Novartis: Consultancy; Stelexis: Consultancy, Current equity holder in private company, Honoraria; Bristol Myers Squibb: Research Funding; GSK: Research Funding; Incyte: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal