GPRC5D-targeted CAR T cell therapy was recently shown to be safe and active in patients with relapsed or refractory multiple myeloma with limited or no other therapeutic options, such as those with triple-refractory disease (to a proteasome inhibitor, an immunomodulatory drug, and an anti-CD38 antibody) and disease progression on a BCMA antibody-drug conjugate, bi-specific T-cell engager, and/or CAR T cell therapy (Mailankody S. et al. NEJM 2022). Among the 17 patients treated in our phase 1 study of the GPRC5D-targeted CAR T cell therapy MCARH109, 12 patients had an objective response and 6 patients relapsed after an initial response of 3-9 months. While there are currently no validated clinical assays to assess GPRC5D expression, we reported decreased or loss of protein expression using immunohistochemistry in all 6 patients who relapsed. This finding suggests that genetic alterations may be an important mechanism of antigen escape after GPRC5D-directed CAR T cell therapy. With BCMA-directed CAR T cell therapies recently approved for multiple myeloma, antigen loss due to genetic alterations is a rare mechanism of resistance.
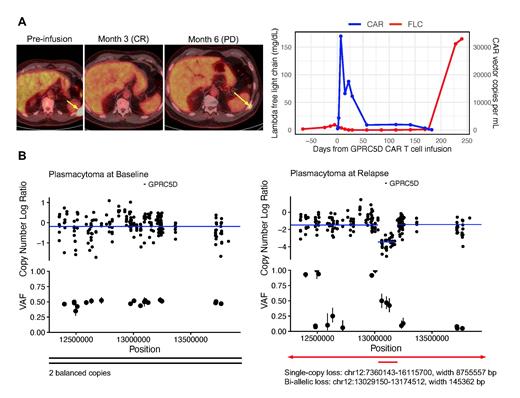
We first investigated the genetic status of GPRC5D in a patient (Patient 15) who underwent biopsies of a left posterior chest wall plasmacytoma at baseline and a recurrent plasmacytoma at the same site at relapse with nearly 100% tumor. This patient received the maximum tolerated dose of 150×10 6 GPRC5D-directed CAR T cells and achieved a best response of stringent complete response without minimal residual disease in the bone marrow. Unfortunately, 6 months after infusion, he relapsed with recurrent FDG-avid lesions in similar locations on PET, extensive bone marrow involvement (90-95%), rapidly rising lambda free light chain in the serum, and significantly decreased but still detectable circulating CAR T cells (CAR vector copies per mL: 666) ( Figure A). We performed droplet digital PCR (ddPCR) on extracted DNA from the plasmacytoma biopsies and estimated GPRC5D copy number to be two at baseline and 0.1 at relapse, consistent with bi-allelic loss in a significant majority of plasma cells. We then performed whole exome sequencing which revealed two balanced copies of GPRC5D at baseline and bi-allelic loss at relapse consisting of a broader deletion (~8756 kb) in chromosome 12p in one allele and a more focal deletion (~145 kb) encompassing the GPRC5D gene locus in the second allele ( Figure B).
Beyond this patient, we identified that relapses after GPRC5D-directed CAR T cell therapy are associated with decreased or loss of GPRC5D mRNA expression. We measured GPRC5D mRNA expression by ddPCR on patient bone marrow or plasmacytoma samples at baseline prior to CAR T cell infusion and at disease progression. The results corresponded with those from immunohistochemistry, and all patients who relapsed showed reduced or absent GPRC5D mRNA and protein expression at disease progression. Given that two patients who responded and relapsed had low or no GPRC5D expression at baseline (Patients 10 and 13), further studies are warranted to characterize the heterogeneity of GPRC5D expression and how well immunohistochemistry and ddPCR capture its expression.
Here we report that antigen escape after GPRC5D-directed CAR T cell therapy can be mediated by structural chromosome alterations resulting in bi-allelic loss of the GPRC5D gene locus in a patient with initial excellent response. We also note transcriptional downregulation of GPRC5D in other patients at relapse, but it remains to be determined whether this is transient or durable and whether it can be explained by similar on-target genetic alterations or non-genetic mechanisms. Our patients with heavily pretreated multiple myeloma may harbor greater tumor heterogeneity and genomic instability than previously described, which can facilitate clonal outgrowth of antigen-negative tumor cells under continuous selective pressure of GPRCRD-targeted CAR T cells. Potential strategies to mitigate antigen escape-mediated relapse in myeloma patients receiving T-cell engaging therapies including earlier use of these therapies, multi-antigen targeting, or combination approaches are being evaluated in ongoing trials.
Disclosures
Mailankody:MJH Life Sciences: Honoraria; Janssen Oncology: Consultancy; Janssen Oncology: Research Funding; Allogene Therapeutics: Research Funding; Bristol Myers Squibb: Research Funding; Physician Education Resource: Honoraria; OncLive: Honoraria; Takeda Oncology: Research Funding; Fate Therapeutics: Research Funding; Optum Oncology: Consultancy; Legend Biotech: Consultancy; Caribou Therapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal