Introduction: The treatment of multiple myeloma (MM) has evolved rapidly, with various combinations of immunomodulators (IMiDs), proteasome inhibitors (PIs), corticosteroids and autologous stem cell transplantation (ASCT) utilized in newly diagnosed (ND) patients; in some jurisdictions, monoclonal antibodies (MABs) are also incorporated in first-line treatment. Over the last decade, the standard of care in Canada for NDMM in fit younger patients has consistently been induction (CyBorD/more recently RVD) followed by ASCT and maintenance lenalidomide (len) until progression. In transplant-ineligible patients, bortezomib- or len-based treatments in doublets or triplets have been used frontline. At relapse, options have included re-induction followed by 2nd ASCT or doublet/triplet combinations until progression. Limited comparative data are available to support any one approach. The aim of this retrospective study was to assess the treatment patterns and outcomes of MM patients treated at 1st relapse in a real-world setting. This data may be useful to myeloma stakeholders when evaluating the potential impact of even newer novel immunotherapeutic agents in patients in who have had 1 prior line of treatment.
Methods: We performed a retrospective observational study using the Canadian Myeloma Research Group Database (CMRG-DB), which is a prospectively maintained disease-specific database with ≥ 9000 patients enrolled from 16 academic sites across Canada. All MM patients who initiated second-line therapy between 01/12/2010 - 30/06/2022 were included and results were analyzed up to 23/05/2023. We aimed to evaluate the following outcomes for each second-line regimen: overall response rate (ORR), progression-free survival (PFS) and overall survival (OS), calculated from the start of second-line therapy. Survival was estimated using Kaplan-Meier methods and compared between groups using the log rank test.
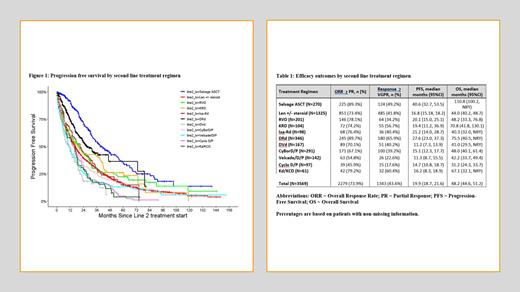
Results: A total of 3569 patients were identified: 1638 (45.9%) who had prior ASCT and 1931 (54.1%) non-ASCT patients. 2715 (76%) patients were bortezomib-exposed in first line, 1052 (30%) were lenalidomide-exposed; 22% had high-risk cytogenetics. The most commonly used second-line regimens were Rd in 1325 (37%) of patients, DRd in 346 (10%), CyBorD in 291 (8%), 2nd ASCT in 270 (8%), RVd in 201 (6%) and DVd in 167 (5%). Outcomes including ORR, ≥ VGPR rate, PFS and OS from second-line treatment for regimens with >50 patients are presented in Table 1. Among all patients, the highest ORRs were seen with DRd (90%), 2nd ASCT (89%), Kd/KCd (79%), RVd (78.1%) and KRd (74%); DRd had the highest ≥ VGPR rate (66%). In patients who had received bortezomib-based first line treatment, the second line ORRs were 96%, 98% and 95% for VRd, KRd and DRd, respectively, with a corresponding median PFS of 20 months (VRd), 19 months (KRd) and 28 months (DRd). For patients who had len-based first line treatment, the second line ORRs were 80% for Kd/KCd and 95% for DVd with a median PFS of 16 months (Kd/KCd) and 11 months (DVd), respectively. The median PFS was 41 months after 2 nd ASCT; of these patients, 68% were exposed to bortezomib in first line and 34% were len exposed in first line. The median PFS was 30 months in those who had len maintenance after their 1 st transplant compared to 60 months in those with no initial maintenance.
Conclusion: In this real-world observational study we demonstrate that Canadian patients achieved results comparable those noted in prospective clinical trials leading to the approval of these agents in the second-line setting. Triplet combinations with an IMID backbone, including DRd, KRd and Ixa-Rd, had high response rates, with DRd offering the longest PFS. Patients undergoing 2 nd ASCT had ORRs and PFS comparable to DRd, although these results were likely influenced by selection of a population with a favorable response to 1st ASCT . Given the expanding use of IMID, PI and MAB combinations in first-line, our results highlight the need for better modalities at the time of 1 st relapse. Earlier integration of “novel” agents, including CAR-T therapy, bi-specifics, conjugated antibodies and CEL-MoDs-even in the second-line setting–is likely required to improve on these outcomes. The current analysis provides efficacy benchmarks to guide their implementation.
Financial Support: CMRG received financial support from Janssen Inc. for the conduct of this study
Disclosures
McCurdy:Amgen: Honoraria; Sanofi: Honoraria; Janssen: Honoraria; GSK: Honoraria; Celgene: Honoraria; Pfizer: Consultancy, Honoraria; Takeda: Honoraria; Forus therapeutics: Consultancy, Honoraria. Reece:Pfizer: Honoraria; BMS: Consultancy, Honoraria, Research Funding; Sanofi: Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Millennium: Research Funding; Amgen: Consultancy; Takeda: Consultancy, Honoraria, Research Funding; GSK: Honoraria. Venner:Sanofi: Honoraria; Forus: Honoraria; AbbVie: Honoraria; GSK: Honoraria; Pfizer: Honoraria; BMS: Honoraria; Janssen: Honoraria. White:GSK: Honoraria; Janssen: Honoraria; Karyopharm: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria; Takeda: Honoraria; Forus: Honoraria; BMS: Honoraria; Antengene: Honoraria; Amgen: Honoraria. Chu:Gilead: Honoraria; Janssen: Honoraria; AbbVie: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria; Amgen: Honoraria; Miltenyi: Research Funding; BMS/Celgene: Honoraria, Research Funding; AstraZeneca: Honoraria. Jimenez-Zepeda:Merck: Honoraria; Takeda: Honoraria; Janssen: Honoraria; Celgene: Honoraria; BMS: Honoraria. Song:Janssen: Honoraria; GSK: Honoraria; Novartis: Honoraria; BMS: Honoraria; Sanofi: Honoraria; Gilead: Honoraria; Amgen: Honoraria; Forus: Honoraria. Mian:Celgene: Honoraria; Janssen: Honoraria; Amgen: Honoraria; Takeda: Honoraria; Sanofi: Honoraria; GSK Awards: HHS Research Early Career Award from Hamilton Health Sciences Foundation: Honoraria. Sebag:Amgen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Bergstrom:BMS: Honoraria, Research Funding; Janssen: Honoraria. Stakiw:Janssen: Honoraria; FORUS Therapeutics: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria. Reiman:Pfizer: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Regeneron: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding. Kotb:Sanofi: Honoraria, Research Funding; Celgene: Honoraria; Pfizer: Honoraria; Takeda: Honoraria; Merck: Honoraria, Research Funding; Janssen: Honoraria; BMS: Honoraria; Karyopharm: Current equity holder in private company; Forus: Honoraria; Akcea: Honoraria; Amgen: Honoraria. Aslam:Celgene: Honoraria; Janssen: Honoraria; Abbvie: Honoraria; Gilead: Honoraria. Kaedbey:Sanofi: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; FORUS Therapeutics: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Louzada:GSK: Research Funding; Forus: Honoraria; BMS: Honoraria; Janssen: Honoraria. LeBlanc:Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; FORUS Therapeutics: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal