Sickle cell disease (SCD) is a genetic disorder caused by a point mutation in the beta-globin gene producing an abnormal hemoglobin (HbS) which, under deoxygenation, polymerizes and causes severe distortion of the red blood cell (RBC). RBCs may be dehydrated, poorly deformable and highly adhesive to the endothelium, with abnormal adhesion being of primary interest to this study.
Recently, several new disease modifying drugs have been FDA approved, with more in development. These novel agents target specific aspects of SCD pathophysiology, including adhesion. Changes induced by such therapies are not always detectable by common conventional lab instruments. LORRCA (oxygen-gradient ektacytometry) is the gold-standard for deformability measurement; it has been well described both analytically and clinically in SCD. Adhesion and deformability have a dynamic, yet complementary relationship that we explore here. While these may behave in an interdependent manner, each determinant must be considered independently.
There is a growing demand for innovative tools to evaluate rheological properties under physiological conditions, particularly microfluidic devices. However, many current devices are limited in their clinical utility due to, choice of ligand, complexity of design, or reliance on endothelial cell culture (typically HUVEC cells) which are difficult to use, vary with passage number, and do not represent adult SCD endothelium.
We developed a Red blood cell Adhesion Chip (RAdChip) to model RBC to endothelial adhesion using laminin, a physiologically relevant ligand, to easily, effectively, and reproducibly measure RBC adhesion. Here, we describe our analytic and clinical validation of our device.
Peripheral blood samples were collected in EDTA from individuals with SCD and healthy controls under an IRB approved protocol. RBCs were extracted via centrifugation, washed and resuspended in PBS to a 0.5% HCT.
Microfluidic devices were fabricated using polydimethylsiloxane (PDMS). A 5ug/ml solution of recombinant laminin was applied to each channel, then blocked with 1% bovine serum albumin (BSA).
Resuspended RBCs were introduced to the device through tubing connected to the inlet port. Harvard Apparatus syringe pumps were used to perfuse samples and wash channels at ~1dyne/cm 2. Adhesion was visualized with a Keyence inverted bright-field microscope at 20x (Itasca, IL). Adhered cells were counted within a 1.8mm 2 region of interest (Roi) using the iCLOTS automated cell quantification software (Fay et al, Nat comm accepted 7/2023).
We measured adhesion index via RAdChip for common SCD genotypes: HbSS/SB0: (median (x͂) =756; range (r): 154-1889; n=26) HbSC/SB+ : (x͂ =299; r=: 114-731; n=11 ) HbAA: (x͂) =13; r= 5-90; n=7). RBC adhesion values were independent of age (p=0.65) and gender (p=0.63).
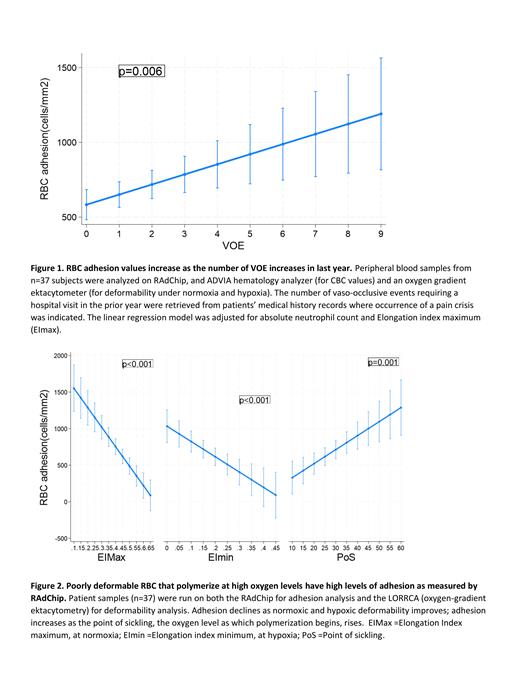
An increase of 0.1 unit in EImin and EImax decreases RBC adhesion by 214 and 268 cells/mm 2, respectively. A 1mmHg increase in PoS increased adhesion by 19.5 cells/mm 2. sRBC adhesion had a significant positive association with vaso-occlusive events (VOE) (p=0.005), absolute neutrophil count (ANC) (p=0.005), and EImax (p<0.001).
Notably, analytical validation of RAdChip produced a mean CV of 0.13 ± 0.94.
RAdChip adds a key component of functional RBC evaluation that conventional lab equipment does not measure. We have analytically validated our device, demonstrating an excellent coefficient of variability. 44 patient samples, each examined in triplicate produced a mean CV of 0.13.
We have demonstrated clinical validity of our device by establishing correlations with known markers of severity and pain event frequency. RBC adhesion values increase as the number of VOE events increase, reaffirming the belief that adhesion is a principal element in the multistep VOE cascade. In like manner, improvements in deformability are associated with decreased RBC adhesion, strengthening the interdependent nature of VOE molecular events.
Recognizing the dynamic nature of SCD, it is necessary to explore sickle erythrocyte adhesion longitudinally to investigate changes before, during, and after VOE occurrences. Continued and increased integration of adhesion measurements with RAdChip and other rheology devices into routine SCD care may be helpful in assessing disease severity, identifying need for additional disease modifying agents, and assist with second line agent selection and monitoring.
Disclosures
Lam:Cellia Science, Inc: Current equity holder in private company; Sanguina, Inc: Current equity holder in private company. Sheehan:Refoxy Pharmaceuticals: Research Funding; Afimmune: Research Funding; Pfizer Inc: Research Funding; Novartis: Research Funding; Beam Therapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal