Introduction
Rare non-hematopoietic cells in the bone marrow make essential contributions to hematopoiesis. Despite tremendous progress in understanding the mouse bone marrow microenvironment, the precise identity of human non-hematopoietic bone marrow cells and their spatial organization remain largely uncharacterized. Here, we performed single cell transcriptomics and spatial proteomics, assembling the first comprehensive atlas of human bone marrow cellular composition and organization.
Methods
Using fresh femoral head samples obtained from orthopedic hip replacement surgeries, we first performed single-cell RNA sequencing (scRNA-Seq) to create a comprehensive human bone marrow atlas profiling 29,325 enriched non-hematopoietic cells as well as 53,417 hematopoietic cells from enzymatically digested femoral heads from 12 individuals. We next employed Co-Detection by Indexing (CODEX) multiplexed imaging of 18 bone marrow samples, including both healthy and acute myeloid leukemia (AML) samples, to spatially profile over one million single cells with a novel 53-antibody panel.
Results
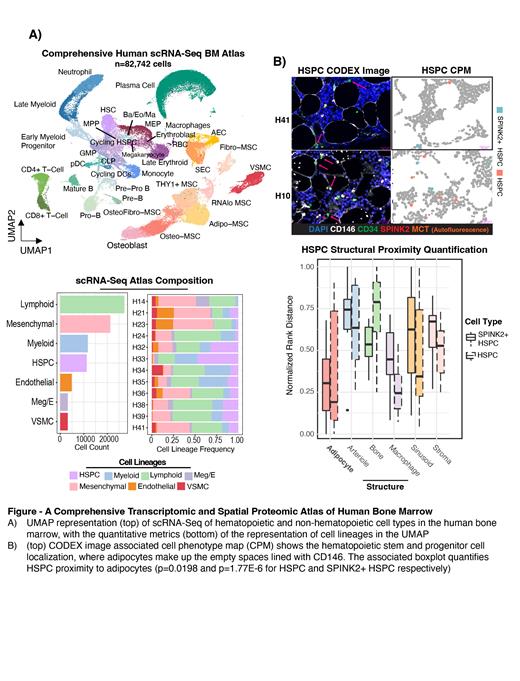
Our transcriptional analysis revealed nine distinct non-hematopoietic bone marrow cell types including mesenchymal stromal cell (MSC) subsets, osteolineage cells, vascular smooth muscle cells, and both sinusoidal and arterial endothelial cells (Figure 1A). We found that MSCs were heterogeneous and hierarchically organized. One specific cell cluster, which we termed Fibro-MSCs, corresponded to previous descriptions of mesenchymal stem cells and was both computationally and functionally shown to be the most stem/progenitor-like. Next, we performed computational cellular communication analysis which revealed that two MSC subsets, Adipo and THY1+ MSCs, provided the majority of canonical hematopoietic supportive factors such as CXCL12, KITLG, IL7, and PTN.
Next, we used CODEX to systematically study spatial relationships between these cells. Consistent with the scRNA-Seq cellular communication analysis, we found that Adipo and THY1+ MSCs were in the bone marrow interacting with hematopoietic cells, while osteolineage cells and Fibro-MSCs were found within the trabecular bone region. We then performed unsupervised neighborhood analysis to analyze the distinct niches of the bone marrow. We discovered a relatively hyperoxygenated arterio-endosteal niche for early myelopoiesis, where early myeloid progenitors were characterized by very low levels of HIF1α, in contrast to HIF1α hi neutrophils which were found largely residing near sinusoids. We also employed point pattern statistics and permutation tests to analyze the proximity of cell types to manually identified structures in the bone marrow. This analysis revealed that CD34+ hematopoietic stem and progenitor cells (HSPCs) were frequently contacting adipocytes (Figure 1B), but not sinusoids or bone. Collectively, these results led us to propose a data-driven working model for how human myelopoiesis is spatially organized, with the earliest myeloid progenitors being specified from HSPCs near adipocytes, then migrating to the peri-arteriolar/peri-endosteal early myeloid progenitor niche, and then finally localizing near sinusoids which facilitates bone marrow egress as they mature to neutrophils.
To evaluate if our CODEX atlas could be used to study disease states, we mapped new bone marrow images from acute myeloid leukemia (AML) patients and controls to our reference. We used machine learning to identify true mutant cells based on NPM1c expression patterns. We discovered Adipo and THY1+ MSC expansion in AML patients compared to controls, with expanded MSCs spatially associated with leukemic blasts. Our results highlight the potential of our CODEX atlas to contextualize new datasets and study bone marrow organization in disease states, and provide further rationale to study MSC-AML blast interactions.
Conclusions
Taken together, our scRNA-Seq and CODEX data act in concert, demonstrating the diversity of non-hematopoietic stromal elements, establishing the source of hematopoietic cytokines, and defining the spatial organization in healthy human bone marrow. We envision our multiomic, spatially resolved atlas of human bone marrow will serve as a critical resource for future study of the bone marrow microenvironment in both healthy and disease states.
Disclosures
Maillard:Garuda Therapeutics: Membership on an entity's Board of Directors or advisory committees; Regeneron: Research Funding; Genentech: Research Funding. Carroll:Cartography Bioscences: Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceuticals: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal