Introduction. The prognosis of Ph+ acute lymphoblastic leukemia (ALL) has changed profoundly following the advent of tyrosine kinase inhibitors (TKIs). Since 2000, the GIMEMA frontline studies for adult Ph+ ALL patients have been based on a TKI plus steroids in induction without systemic chemotherapy. This has allowed complete hematologic remission (CHR) rates of 94-100%, irrespective of age, with virtually no deaths in induction 1-5. We then designed an induction/consolidation chemotherapy-free frontline trial with dasatinib followed by blinatumomab (GIMEMA LAL2116, D-ALBA). The preliminary results 6,7 showed a CHR rate of 98% with 60% of molecular responses at the primary endpoint of the study (after 2 blinatumomab cycles); the OS and DFS were 95% and 88% at 18 months. Here we present the final long-term results of the study.
Methods. Post-consolidation treatment was left to the treating physicians and all the information has been collected in the GIMEMA LAL2217 ancillary study. Sixty-three patients were enrolled (median age 54 years, range 24-82); 2 patients withdrew from the trial, 1 died in CHR and 1 relapsed after induction due to a major protocol violation 6,7.
Results. After the primary endpoint, molecular responses increased further following additional blinatumomab cycles, being 75, 77, 70, 85, 90 and 93% at 2, 4, 6, 8, 10 and 12 months from the last blinatumomab cycle. At a prolonged follow-up, a persistent molecular negativity was found with 78.3, 73.1, 85, 87.4, 94.2, 90.2, 86.6 and 86.7% of responders at 16, 20, 24, 28, 32, 36, 42 and 48 months in the evaluable population.
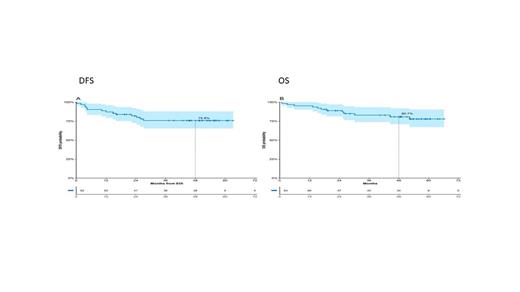
At a median follow-up of 53 months, DFS and OS are 75.8% and 80.7% (Figure 1). A significantly better DFS and OS was observed in the 17 patients in molecular response at the end of induction (EOI) (100%) vs cases who were not (42) (68.6%) (p=0.016 and 0.036, respectively). These patients have so far not relapsed. After 2 blinatumomab cycles, DFS and OS did not differ between molecular (33) and non-molecular (22) responders: 82.9% and 89.4% vs 75.7% and 84.2%, underlying the effect of blinatumomab. A worse DFS from the EOI was recorded for IKZF1 pluspatients (11; 45.5%) compared to those without IKZF1 deletion (25; 82.3%) or with IKZF1 only deletions (13; 74%) (p=0.029). Similar results were observed also for OS. A similar trend was observed also when DFS was calculated from the primary endpoint of the study (62.5% vs 84.8% vs 74%). The 4 patients with a concomitant IKZF1 plus signature and T315I mutation had the worse outcome.
After induction/consolidation, 29 patients continued treatment only with a TKI; 27 (93.1%) had obtained a deep molecular response after dasatinib/blinatumomab. Overall, 28 patients (96.5%) who did not receive systemic chemotherapy/transplant are in persistent CHR at a median follow-up of 48 months. Of the 24 patients transplanted in 1 st CHR, 13 (54.2%) were non-molecular responders at the primary endpoint and only 2 became molecularly negative after further blinatumomab. Twenty patients (83.3%) are alive in CHR at a median follow-up of 49 months, 1 has relapsed 1 month after the transplant and 3 have died of complications. Three/6 patients transplanted in 2 nd CHR are alive at 30, 40 and 52 months. The transplant-related mortality was 12.5% for patients transplanted in 1 st CHR and 13.7% overall.
Nine relapses have occurred: 4 hematologic, 4 involving the CNS and 1 nodal. The median time to relapse was 4.4 months (1.9-25.8). A IKZF1 plus signature, evaluated at diagnosis, was present in 4/8 evaluable cases. ABL1 mutations, evaluated on BM samples at relapse or at a concomitant MRD increase in extra-hematologic recurrences, were found in 7 cases.
Treatment was well tolerated with no unexpected long-term toxicities.
Conclusions. The final analysis of the D-ALBA study shows that a chemotherapy-free induction/consolidation regimen based on a targeted strategy and immunotherapy is feasible and effective in inducing durable long-term hematologic and molecular responses in Ph+ ALL patients of all ages. Half of the patients remained only on a TKI and never underwent systemic chemotherapy or a transplant. These results pave the way for a new era in the treatment of adult Ph+ ALL patients.
1. Vignetti et al, Blood 2007
2.Foà et al, Blood 2011
3. Chiaretti et al, Haematologica 2016
4. Chiaretti et al, Haematologica 2021
5. Martinelli et al, Blood Advances 2022
6. Foà et al, N Engl J Med 2020
7. Foà & Chiaretti, N Engl J Med 2022
Disclosures
Ferrara:ABBVIE: Honoraria. Mulè:ABBVIE: Honoraria; Incyte: Honoraria; Pfizer: Honoraria; Astellas: Honoraria. Bonifacio:Novartis: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Clinigen: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees. Fracchiolla:Abbvie, Jazz, Pfizer, Amgen: Other: travel grants; Abbvie, Jazz, Pfizer, Amgen: Speakers Bureau. Rambaldi:Abbvie: Honoraria. Chiaretti:Abbvie: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal