Background: Congenital thrombotic thrombocytopenic purpura (cTTP) is an ultra-rare, thrombotic microangiopathy due to an inherited deficiency of the von Willebrand factor (VWF)-cleaving protease, ADAMTS13.Accumulation of ultra-large VWF multimers increases blood clot formation in small blood vessels, leading to a low platelet count (thrombocytopenia). Treatment of cTTP consists of ADAMTS13 replacement with infusions of plasma-based therapies (PBT). Recombinant ADAMTS13 (rADAMTS13; Takeda Development Center Americas, Inc., Lexington, MA, USA) is in development for patients with cTTP.The ultra-rare nature of cTTP limits the sample size for a formally powered study to assess the impact of ADAMTS13 supplementation on efficacy outcome. This study was a proof-of-principle that model-based clinical trial simulations can be used to supplement clinical trial data in ultra-rare indications.
Aims: To calibrate and validate a quantitative system pharmacology (QSP) model describing a mechanistic relationship between ADAMTS13, VWF and platelet numbers in patients with cTTP who received long-term prophylaxis with rADAMTS13 or PBT (fresh frozen or solvent/detergent treated plasma). In addition, to evaluate the treatment benefits of rADAMTS13 versus PBT using clinical trial simulations in virtual patients with cTTP as a supplement to the cTTP phase 3 study (NCT03393975) data.
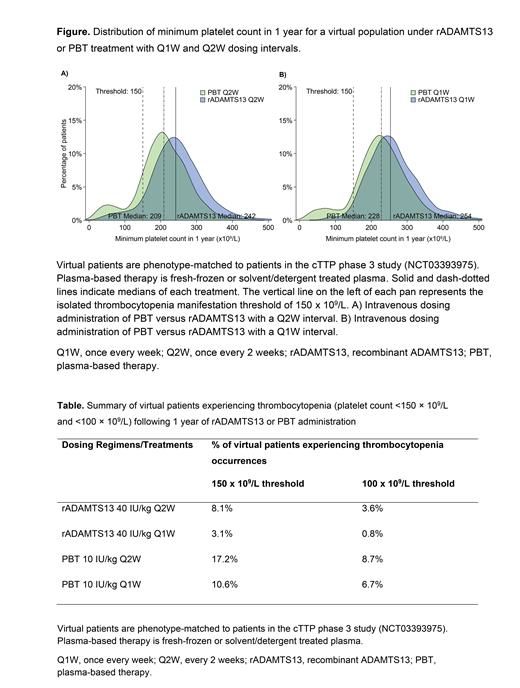
Methods: A QSP model was built using i) in vitro enzyme kinetic data and preclinical experimental data following administration of recombinant VWF and rADAMTS13 in ADAMTS13 knockout (KO) mice, and ii) data from the literature including platelet count and VWF activity from healthy human and untreated patients with cTTP. These data were used to calibrate the parameters corresponding to interactions between ADAMTS13 activity, VWF activity, and platelets. The model was validated against literature data from patients with cTTP treated with PBT and with data from a phase 2 study of patients with immune-mediated TTP treated with rADAMTS13 (NCT03922308). The model was then calibrated to longitudinal platelet data from the cTTP phase 3 study, in which patients with cTTP received prophylaxis for 6 months with PBT (exposure equivalent to ~10 IU/kg ADAMTS13 in the form of 10-20 mL/kg doses) and rADAMTS13 (40 IU/kg) either once every week (Q1W) or once every 2 weeks (Q2W) dosing intervals . The model was used to provide 1-year clinical trial simulations of isolated thrombocytopenia occurrences in a population of 1,000 virtual patients, phenotype-matched to patients in the cTTP phase 3 study.
Results:Virtual clinical trial simulations using the QSP model suggested that rADAMTS13 administered over 1 year with a Q1W or Q2W schedule results in significantly fewer patients experiencing occurrences of thrombocytopenia (platelet count threshold of <150 x 10 9/L) compared to patients treated with PBT (hazard ratio [HR] relative to Q2W PBT: Q1W PBT, HR = 0.62; Q2W rADAMTS13, HR = 0.47; Q1W rADAMTS13, HR = 0.18). The treatment difference between rADAMTS13 and PBT was even more pronounced when a thrombocytopenia threshold of <100 x 10 9/L was used (Figure and Table). Simulations also predicted a prophylactic carry-over effect on platelets, with approximately 20 days taken to reach 90% of the long-term average platelet count when switching from rADAMTS13 to PBT or from PBT to rADAMTS13. A correlation analysis for a Q2W dosing of rADAMTS13 demonstrated that ADAMTS13 trough activity (C trough, Spearman ρ = 0.96) and time with ADAMTS13 activity above 10% (Spearman ρ = 0.93) showed stronger correlations with increased platelet count than the maximum plasma ADAMTS13 activity level (C max, Spearman ρ = 0.68). A correlation analysis for a Q1W dosing frequency produced similar results.Mean time above 10% ADAMTS13 activity was higher following rADAMTS13 versus PBT administration (5.2 vs 1.7 days).
Conclusions: This model-based virtual clinical trial simulation predicts that dosing with rADAMTS13 would provide >50% reduction in the HR of thrombocytopenia occurrences over PBT. Patients may also have a higher level of protection for 2-3 weeks when switching from rADAMTS13 to PBT. These findings supplement the assessment of clinical benefit of rADAMTS13 in the cTTP phase 3 study and show that increased ADAMTS13 activity following rADAMTS13 administration is likely to reduce occurrences of thrombocytopenia in cTTP.
Disclosures
McBride:Takeda Development Center Americas Inc.: Current Employment, Current equity holder in publicly-traded company. Jiang:RES Group Inc.: Current Employment. Zhang:RES Group Inc.: Current Employment. Tolsma:RES Group Inc.: Current Employment. Mellgård:Takeda Development Center Americas, Inc.: Current Employment, Current equity holder in publicly-traded company. Vakilynejad:Takeda Development Center Americas Inc.: Current Employment, Current equity holder in publicly-traded company. Bhattacharya:Takeda Development Center Americas Inc.: Current Employment, Current equity holder in publicly-traded company. Zhu:Takeda Development Center Americas Inc.: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal