Introduction. Infections are a major source of morbidity and mortality in patients with Chronic Lymphocytic Leukemia (CLL). The development of targeted agents decreased the rate of these complications in comparison to standard chemoimmunotherapy regimens. However, these patients often elderly, with other comorbidities, heavily treated, experienced serious infections.
The aim of our study was to evaluate the incidence of clinically or microbiologically documented bacterial, fungal and viral infectious complications in CLL patients treated with venetoclax.
Methods. The retrospective multicenter study included CLL patients treated since 2017 with venetoclax single agent until progression or toxicity or venetoclax plus anti-CD20 antibody (mainly rituximab as part of VR protocol for 24 months or obinutuzumab as part of VO protocol for 12 months).
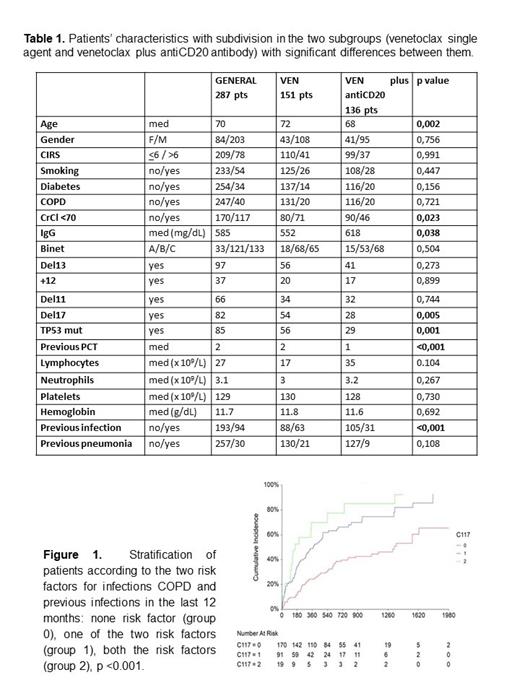
Results. A total of 287 patients with CLL received venetoclax during the study period from 16 different institutions: 151 patients (52.6%) as monotherapy and 136 (47.4%) associated to anti-CD20 antibody. Basal characteristics of the whole population and of the two groups are summarized in Table 1. Patients of the first group were older, more frequently had del17/TP53mut, renal impairment and lower basal levels of IgG. They also showed more previous infections in the 12 months before the beginning of the treatment with venetoclax.
We registered 284 infections of any grade. When comparing time of first infection between the patients treated with venetoclax and those treated with venetoclax plus anti-CD20 antibody, we registered a trend toward a higher rate of infection in the latter group after the first year (p=0.066). This difference was not confirmed when we focused on infections of grade 3-4 (p=0.521).
One-hundred eighty-one infections of grade 1-2 developed in 114 patients (39.7%) during the study. Most of the infections involved the respiratory tract (106 events, 58.6%), followed by genitourinary tract (23, 12.7%) and gastrointestinal one (16, 8.8%). Pathogens implicated in the infections were isolated only in 57 (31.5%) cases: 36 viral, 18 bacterial and 3 fungal. We recorded 103 episodes of infections of grade 3-4, occurred in 73 patients (25.4%). The most common site of infection involved the respiratory tract (71 events, 68.9%), then we registered sepsis (13, 12.6%) and gastrointestinal tract infections (7 events, 6.8%). Of 103 severe infections, 64 (62.1%) were microbiologically proven, of whom 40 were viral, 21 bacterial and 3 fungal.
When comparing patients with and without infection, COPD (p<0.001, OR 3.75), previous infections in the last 12 months (p<0.001, OR 3.15), renal impairment CrCl<70 (p = 0.049, OR 1.62), previous treatments (p=0.023; OR 1.196) and stage A (p=0.001; OR 0.2) were more frequently associated with infection in univariate analysis. In multivariate analysis COPD (p <0.001, OR 5.39) and previous infections (p=0.001, OR 2.57) resulted significant. Stratifying patients according to COPD and previous infections in the last 12 months we obtained 3 groups significantly different in terms of infective risk (p <0.001; figure 1). When considering only grade 3-4 infections, risk factors significant in the univariate analysis were COPD (p <0.001, OR 3.23), smoke (p=0.033, OR 1.98) and previous infections (p=0.020, OR 1.91). COPD was the unique significant variable in multivariate analysis (p=0.008, OR 2.62). Treatment was withdrawn for infections in 80 patients (27.9%): in 58 (20.2%) treatment was temporarily discontinued, while in 22 (7.7%) discontinuation was permanent. The infections that caused definitive withdrawals were mainly pneumonia (12 cases, 6 of whom from SarS-CoV2 infection) and sepsis (8 cases, 5 of whom after a SarS-CoV2 infection). A total of 83 patients (28.9%) died and the median OS was 55 months. The main causes of death were CLL progression in 36 cases and infection in 22 cases.
Conclusions. This is a real-life study on 287 patients affected by LLC treated with venetoclax with the aim to describe the infectious complications in such population in routine clinical practice.
The analysis found a significant rate of infections, most of grade 1-2: 39.7% of the patients experienced a grade 1-2 infection; 25.4% a grade 3-4 infection. The identification of additional infectious risk factors found a role of comorbidities such as COPD and previous infections; COPD resulted a risk factor also for infections of grade 3-4.
Disclosures
Visentin:Janssen: Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; CSL behring: Membership on an entity's Board of Directors or advisory committees; Takeda: Speakers Bureau; BeiGene: Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding. Vitale:Janssen, Abbvie, Astra-Zeneca, Beigene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Sanna:Janssen: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Astrazeneca: Consultancy, Speakers Bureau. Sportoletti:Abbvie, Janssen, Beigene, Astra Zeneca, Takeda, Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Tedeschi:Astrazeneca: Speakers Bureau; Abbvie: Speakers Bureau; Beigene: Speakers Bureau; Janssen: Speakers Bureau. Candoni:Pfizer: Consultancy; Astellas: Honoraria; Janssen: Honoraria; Incyte: Consultancy, Honoraria. Pagano:Novartis: Honoraria; Pfizer: Honoraria; Gilead: Honoraria; Janseen: Honoraria; AstraZeneca: Honoraria; Moderna: Honoraria; Jazz: Honoraria; Menarini: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal