Background
CD19-directed CAR-T cell therapy is a valuable treatment option for relapsed/refractory B-cell non-Hodgkin's lymphoma (r/r B-NHL). It has been shown that a significant percentage of B-NHL clinical samples also express B cell maturation antigen (BCMA). To further improve safety and efficacy, we have been exploring utility of a CD19 and BCMA dual-targeting CAR-T, GC012F, manufactured through a novel next-day FasTCAR-T process, for treatment of r/r B-NHL. Here, we present updated results of this ongoing IIT trial with more patients and longer follow-up.
Methods
From October 2021 to June 2023, nine eligible refractory/ relapsed defuse large B cell lymphoma (r/r DLBCL) patients (pts) were enrolled in this study and treated with a single infusion of GC012F in escalating dosing cohorts after a standard lymphodepletion regimen over 3 days (20 - 30 mg/m 2/day Flu, 250 - 300 mg/m 2/day Cy). The primary objectives of this study were safety and tolerability assessment; the secondary objectives were pharmacokinetics and efficacy. Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were graded by ASBMT 2019, and adverse effects were evaluated according to CTCAE 5.0. Efficacy assessment of GC012F was based on the Lugano criteria. Expansion of CAR-T cells and CAR copy numbers were analyzed by flow cytometry and qPCR, respectively.
Results
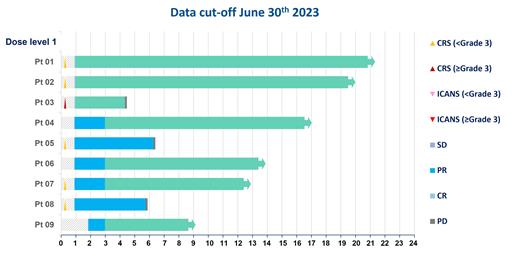
As of data cut-off of June 30 th, 2023, with a median of 372 days (range 131 - 625) of follow-up, all nine patients received single GC012F infusions at dose levels from 3.7×10 4 to 3×10 5 CAR-T/kg, and completed 3 months or longer time follow-up evaluations. The median age was 52 years (range 18 - 60). Among the enrolled patients, eight pts were Ann Arbor stage III/IV disease, one patient with double hit, and four pts had IPI score of 3. The lymphoma samples of all patient expressed CD19, and 7 out of 8 expressed various levels of BCMA. The median SPD was 2690.81 mm 2 (408.3 - 13325.96 mm 2). Patients received a median of 2 prior lines (range 2 - 3) of therapy including rituximab and anthracyclines. Two patients received prior auto-HSCT. All patients responded to GC012F treatments, 100% (9/9) pts achieved ORR at month 3 post GC012F infusion; 77.8% (7/9) CR at month 3, 66.7% (6/9) CR at month 6 and 62.5% (5/8) CR at month 9, respectively. Up to date, the longest duration of remission was 20.8 months. No dose-limiting toxicities were observed. Five patients experienced grade 1 CRS, and one patient in the highest dose group (3×10 5 CAR-T/kg) had grade 3 CRS within 2 days. No ICANS were observed. Of the nine treated patients, grade ≥ 3 hematologic toxicities were neutropenia (9/9), leukopenia (8/9), lymphocytopenia (8/9), and thrombocytopenia (7/9). All TEAEs were resolved after treatment with standard of care and supportive care. The median peak copy number of CAR-T cells in the peripheral blood was 71,000 copies/μg DNA (range 9263 - 185,039), and the median peak time was 14 days (range 9 - 21d). CAR-T geometric mean AUC 0-28 for patients was 658,538 copies/μg DNA × day (319,104 - 1,359,031; 95% CI). CAR-T cells were also detected in tumor biopsies from all five patients tested.
Conclusion
This first-in-human trial of GC012F, a CD19-BCMA dual targeting CAR-T product, for the treatment of r/r B-NHL showed a manageable safety profile and promising clinical responses. The ORR was 100% at month 3 with 77.8% (7/9) achieving CR. GC012F CAR-T cells were detected in the tumor biopsies, indicating the infiltration of CAR-T cells into the tumor lesions. Overall, based on the safety and efficacy results, CD19-BCMA dual targeting CAR-T product GC012F is a promising therapy for patients with refractory/ relapsed DLBCL.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal