Infants with KMT2A rearranged (KMT2Ar) acute lymphoblastic leukemia (ALL) have a poor prognosis with an event-free-survival (EFS) of 33.6-36.9% on recent Children's Oncology Group and Interfant trials. In preclinical studies, we identified bortezomib and vorinostat as novel active agents in primary KMT2Ar infant ALL specimens that have not been included in infant regimens to date. Total therapy for infants with acute lymphoblastic leukemia I (TINI) evaluated this novel combination in a multi-institutional pilot trial for infants with de novo ALL. In this study, bortezomib and vorinostat were incorporated into an ALL chemotherapy backbone containing dexamethasone, mitoxantrone, and pegasparaginase during induction and reinduction chemotherapy cycles. A run-in dose escalation evaluated 3 dose levels of vorinostat which was then followed by a dose expansion phase. The primary endpoint of the study was tolerability; secondary endpoints included minimal residual disease (MRD), 3-year EFS and overall survival (OS). Fifty patients enrolled between 2017-2021. Median follow up was 3.4 years (range 1.6-6.8 years). The six patients in the dose escalation phase had no dose-limiting toxicities. Forty-four patients received vorinostat at the third dose level (DL3) of 180 mg/m 2/dose. 68% of patients treated at DL3 had CNS involvement at diagnosis. 68% of DL3 patients carried a KMT2Ar. The most frequent KMT2A partner genes were AFF1 (40%), MLLT1 (20%), and MLLT3 (13%). The most frequent grade 3-4 non-hematologic adverse events during induction include neutropenic fever (45%), hypertension (43%), infection (40%), anorexia (32%), diarrhea (18%) and perineal skin ulceration (16%). There were 3 episodes of sepsis and 1 induction death due to parainfluenza pneumonia. Fewer events and no deaths or proven sepsis occurred during reinduction, with only grade 3 hypertriglyceridemia exceeding 10% of patients affected (18%). 79% of all patients and 69% of KMT2Ar patients became MRD negative by flow cytometry on day 22 of induction following our investigational block. Following induction intensification with cyclophosphamide, cytarabine, and mercaptopurine, 77% and 66% of all patients and KMT2Ar patients respectively were MRD negative at the time of count recovery. Eight of the forty-four patients, all KMT2Ar, underwent stem cell transplant in first remission due to persistent MRD positivity at the end of consolidation.
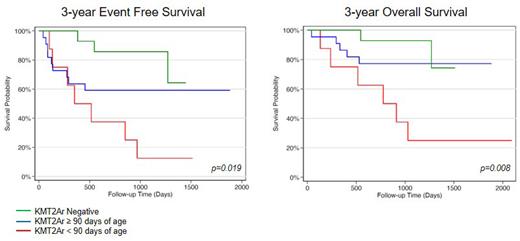
The 3-year EFS and OS probabilities (SE) of all forty-four DL3 patients were 56.5% (8.0) and 70.5% (7.3), respectively. The 3-year cumulative incidence of relapse (CIR) with death considered a competing event was 29.3% (6.9) and the cumulative incidence of death (CID) as a first event was 8.9% (4.5). Patients lacking a KMT2Ar had superior outcomes with 85.7% (9.4) EFS and 92.9% (6.9) OS. The EFS and OS of KMT2Ar patients were 44.8% (9.5) and 62% (9.1) respectively. CIR and CID for KMT2Ar patients was 43.5% (9.6) and 7.2% (5) respectively. KMT2Ar patients ≥90 days at diagnosis had superior outcomes compared to their younger counterparts with 59.1% (10.5) EFS and 77.3% (8.9) OS. End of induction MRD was predictive of an event in this subgroup, with 85.6% (9.4) EFS for MRD negative patients and 14.3% (13.2) EFS for MRD positive patients. In contrast, KMT2Ar patients < 90 days of age at diagnosis had poor outcomes irrespective of MRD with 12.5% (11.7) EFS and 25% (15.3) OS. Notably KMT2Ar patients < 90 days who were MRD negative at the end of induction had a median time to relapse of 600 days (range 282-969 days), a late time point for this malignancy that typically relapses within a year from initial diagnosis. This suggests the regimen effectively reduced tumor burden leading to an extended duration of remission but failed to sufficiently eradicate disease to prevent relapse.
We conclude that the incorporation of bortezomib and vorinostat for treatment of infants with ALL was well tolerated. MRD responses in KMT2Ar patients were robust. Although the study was not powered to evaluate outcomes, EFS and OS in KMT2Ar patients suggest a clinical signal worth pursuing given the low proportion of patients transplanted in first remission. The successor study, TINI 2 (NCT05848687), will build upon the bortezomib/vorinostat backbone by incorporating 2 cycles of blinatumomab and the menin inhibitor ziftomenib in combination with chemotherapy during reinduction.
OffLabel Disclosure:
Gruber:Amgen: Other: Clinical Trial Support for Investigator Initiated Study; Pfizer: Other: Clinical Trial Support for Investigator Initiated Study; Kura Oncology: Other: Clinical Trial Support for Investigator Initiated Study; Element Biosciences: Membership on an entity's Board of Directors or advisory committees. Jeha:Amgen: Other: As part of the mission of St. Jude Global Hiroto Inaba, Victor Santana, Sima Jeha and Caitlyn Duffy participate in the Blincyto Humanitarian Access Program and provide in kind support for this program. . Tran:Servier: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria. Pauley:Pfizer: Research Funding. Huynh:Servier: Research Funding. Horton:Takeda: Research Funding.
Bortezomib (FDA approved for multiple myeloma and mantel cell lymphoma) Vorinostat (FDA approved for cutaneous T-cell lymphoma)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal