Complement is elevated in patients with TRALI; Fc-mediated complement activation is critical for murine antibody-mediated TRALI induction.
Complement-dependent murine TRALI is associated with macrophage trafficking from the lungs to the blood and with increased NET formation.
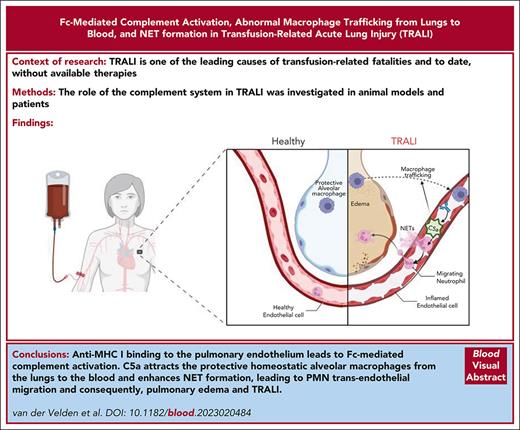
Visual Abstract
Transfusion-related acute lung injury (TRALI) is one of the leading causes of transfusion-related fatalities and, to date, is without available therapies. Here, we investigated the role of the complement system in TRALI. Murine anti–major histocompatibility complex class I antibodies were used in TRALI mouse models, in combination with analyses of plasma samples from patients with TRALI. We found that in vitro complement activation was related to in vivo antibody-mediated TRALI induction, which was correlated with increased macrophage trafficking from the lungs to the blood in a fragment crystallizable region (Fc)-dependent manner and that this was dependent on C5. Human immunoglobulin G 1 variants of the murine TRALI-inducing antibody 34-1-2S, either unable to activate complement and/or bind to Fcγ receptors (FcγRs), revealed an essential role for the complement system, but not for FcγRs, in the onset of 34-1-2S–mediated TRALI in mice. In addition, we found high levels of complement activation in the plasma of patients with TRALI (n = 53), which correlated with elevated neutrophil extracellular trap (NET) markers. In vitro we found that NETs could be formed in a murine, 2-hit model, mimicking TRALI with lipopolysaccharide and C5a stimulation. Collectively, this reveals a critical role of Fc-mediated complement activation in TRALI, with a direct relation to macrophage trafficking from the lungs to the blood and an association with NET formation, suggesting that targeting the complement system may be an attractive therapeutic approach for combating TRALI.
Introduction
The transfusion of blood products is an established and often lifesaving approach in a broad range of clinical settings. Unfortunately, rare life-threatening adverse transfusion reactions, including transfusion-related acute lung injury (TRALI), can occur, which remain difficult to predict and lack therapeutic interventions1,2. TRALI occurs within 6 hours of administration of a transfusion product and remains one of the leading causes of blood transfusion-related fatalities.1,3-6 TRALI clinically manifests as noncardiogenic respiratory distress, observed as pulmonary bilateral infiltrates on chest radiographs.6-9 The pathophysiology of TRALI is complex and involves incompletely understood immunological cellular reactions that lead to pulmonary edema as a result of damaged pulmonary endothelium.10,11 Recipient macrophages and polymorphonuclear neutrophils (PMNs) are known to be essential for the onset of TRALI, whereas cells such as CD4+ regulatory T cells and dendritic cells are protective against TRALI.6,12
TRALI, in general, is seen as a 2-hit model in which the underlying clinical condition of patients (eg, reflected by a state of inflammation) is defined as the first hit and the second hit is delivered by the blood transfusion product, which may contain antileukocyte/endothelial-reactive antibodies or biological response modifiers.1,5,13
It has been suggested that the complement system might be involved in TRALI pathogenesis; however, conflicting results have been reported, which has led to the need for more systematic investigations regarding the contribution of the complement cascade in TRALI.14 It has been shown that the anti–major histocompatibility complex (MHC) class I clone SF1.1.10 (H-2Kd), in contrast to the clone 34-1-2S (H-2Kd; H-2Dd), is not able to induce TRALI in an in vivo murine model.15 In a previous in vitro study we compared both antibodies16 and demonstrated that the antibodies had similar binding capacity to the murine Fcγ receptors (FcγRs), but that 34-1-2S was a more potent activator of complement in vitro.16 To follow up on those observations, we analyzed the involvement of complement in various 2-hit, murine, antibody-mediated TRALI models. In these models, adapted from previously established models,17-19 the first hit consisted of a combination of CD4+ T-cell depletion and priming the animals with lipopolysaccharide (LPS) to induce a mild inflammatory state. The second hit consisted of infusion of the TRALI-inducing antibody 34-1-2S in BALB/c and C5-deficient mice, and a combination of 34-1-2S and AF6.88.5.5.3 in C57BL/6 mice. In addition, we have used advanced antibody engineering and plasma samples from a large cohort of patients with TRALI who fulfilled the 2004 clinical TRALI definitions20 to reveal a critical role for the complement system in TRALI. These data set the stage for pursuing currently unavailable and much warranted therapeutic strategies for TRALI aimed at targeting the complement cascade.
Methods
Human plasma samples
Human TRALI EDTA-plasma samples stored after diagnosis and EDTA-plasma from healthy individuals were used according to the rules of our institute (Sanquin Diagnostic Services, the national reference center for TRALI diagnostics, Amsterdam, The Netherlands). Patient samples fulfilled TRALI criteria according to the 2004 clinical TRALI definitions20 and are described in detail in supplemental Methods, available on the Blood website.
Mice
Wild-type (WT) BALB/c (BALB/cAnNRj; H-2Kd) and WT C57BL/6 (C57Bl/6NRj; H-2Kb) mice were obtained from Janvier Laboratories (Le Genest-Saint-Isle, France). C5-deficient mice (C57BL/6 background with H-2Kd) were purchased from The Jackson Laboratory, Bar Harbor, ME (stock no. 000461). All animal experiments were approved by the Central Netherlands Committee for Animal Testing (ethical permit number AVD3010020198844) and the local animal ethics committee (The Netherlands Cancer Institute, Amsterdam, the Netherlands) and performed in accordance with the Dutch Act on Animal Experimentation (November 2014).
Anti–MHC class I antibodies, Fc deglycosylation, and Fab production
Anti–MHC class I mouse monoclonal immunoglobulin G2a (IgG2a), clones 34-1-2S (H-2Kd; H-2Dd), SF1.1.10 (H-2Kd), AF6-88.5.5.3 (H-2Kb), and the isotype control antibody, clone C1.18.4, were purchased from Bio X Cell (West Lebanon, NH). Antibody deglycolysation was performed with EndoS (deGlycIT kit, Genovis, Lund, Sweden), and fragment antigen-binding region (Fab) fragments were produced with FabULOUS (Genovis) according to the manufacturer’s protocols. Conformation of produced products was performed by (non)reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis and mass spectrometry (data not shown).16
Production of recombinant humanized 34-1-2S variants
The production of chimeric 34-1-2S variants (with silenced complement and/or Fc-receptor function) and the isotype control antibody (anti-2,4,6-trinitrophenyl [TNP]) was performed in a human embryonic kidney 293F production system, as previously described in detail.21,22 Antibody production is described in supplemental Methods.
Antibody-mediated murine TRALI models
TRALI was induced using a 2-hit model adapted from previously established mouse models.17,18,23 As a combined first hit, protective CD4+ T cells were depleted with 4.5 mg/kg anti-CD4 (clone GK1.5, Bio X Cell) intraperitoneally, and mice were primed with 0.1 mg/kg LPS (Escherichia coli O55:B5; Sigma-Aldrich, St. Louis, MO) intraperitoneally 16 hours before initiation of the second hit with specified antibodies, clones 34-1-2S or SF1.1.10 IV for the BALB/c experiments, and a combination of a high dose of 34-1-2S in combination with clone AF6-88.5.5.3 in C57BL/6NRj, as described previously,18 or the isotype control C1.18.4, all at indicated doses of 1 mg/kg, 4.5 mg/kg, or 45 mg/kg. Difference in antibody doses were based on titration experiments (supplemental Figure 1). The C5aR antagonist DF2593a (Calbiochem, Darmstadt, Germany), was injected IV at 10 mg/kg simultaneously with 34-1-2S in 1% dimethyl sulfoxide/phosphate-buffered saline (PBS). Rectal temperatures were determined every 30 minutes using a RET-3 rectal probe (AD Instruments Ltd, Oxford, United Kingdom). Mice that survived till the end point of 90 minutes after TRALI initiation were euthanized with a mixture of ketamine and xylazine. Mice that did not survive until the end point were still included in the analyses.
Lung W/D weight ratios
Lung wet-to-dry (W/D) weight ratios of the right lung lobe were calculated with the formula net wet weight divided by the net dry weight, after a 48-hour incubation at 60°C, as described previously.24
Bronchoalveolar lavage and total protein determination
Bronchoalveolar lavage fluid (BALF) was obtained by excision of the trachea followed by insertion of a 21G lavage tube. PBS (1 mL) was used to flush the lungs twice. Mice were used to either obtain BALF or determine the lung W/D weight ratio. BALF was centrifuged at 2500g for 15 minutes, and protein concentration of supernatant was determined using a Nanodrop 2000 instrument (Thermo Fisher Scientific, Waltham, MA). Flow cytometric analysis were performed on the pelleted cells, as described hereafter.
Blood and tissue processing for flow cytometric analyses
Single-cell suspensions were made by manual homogenization of the lung tissues in 10% citrate-phosphate-dextrose solution with adenine/PBS (Merck Life Science NV, Amsterdam, The Netherlands), followed by transfer of the cells through a 40-μm cell strainer (Corning, Wiesbaden, Germany). Red blood cell lysis was performed with ammonium chloride/potassium lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, and 0.1 mM Na2EDTA.2H2O, Merck). Cell surface Fc receptors were blocked with a CD16/CD32 blocking antibody, followed by staining with the antibody cocktail described in supplemental Table 1. After washing, CD115+ cells were stained with streptavidin allophycocyanin. CD4 T-cell depletion was verified for spleen cells by flow cytometry on a fluorescence-activated cell sorter Canto II (BD Biosciences, Franklin Lakes, NJ).
PMN chemotaxis assay
PMNs were isolated from C57BL/6NRj mice as previously described.25 After labeling for 45 minutes with 50 μg of Calcein AM (Molecular Probes, Eugene, OR), cells were washed and centrifuged at 437g for 5 minutes. As a positive control, macrophage inflammatory protein 2 (10 nM; BioLegend, San Diego, CA) was used. PMNs were primed with LPS for 45 minutes, followed by incubation with recombinant mouse complement protein C5a. (10 nM; BioLegend) for 2 hours. Continuous measurements were performed with a Tecan Infinite 200 PRO plate reader (Tecan, Männedorf, Switzerland) at 485 nm.
NET induction in vitro
PMNs were isolated from C57BL/6NRj mice as previous described.25 PMNs (150 000 per well) were adhered on gelatin-coated coverslips. To induce neutrophil extracellular trap (NET) formation, ionomycin (5 μM, EMD Millipore Corporation, Danvers, MA), LPS (100 ng/mL, Sigma-Aldrich), and C5a (10 nM, R&D Systems, Minneapolis, MN) were diluted in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer. To mimic the human TRALI setting, PMNs were primed with LPS (100 ng/mL) for 1 hour, followed by C5a incubation. After 16 hours, 2% paraformaldehyde (Merck, Darmstadt, Germany) fixation was followed by blocking with 1% bovine serum albumin/PBS (Sigma-Aldrich) for 30 minutes, followed by consecutive incubation with 0.2 μg/mL anti–citrullinated histone H3 (CitH3) (Abcam, Cambridge, United Kingdom) and the secondary antibody goat anti-rabbit AF633 (5 μg/mL; Invitrogen, Waltham, MA) in combination with 5 μM SYTOX Green Nucleic Acid Stain (Invitrogen) for 1 hour. Formed NETs were visualized with a wide-field microscope (Nikon Eclipse Ti2; Nikon Instruments Inc, Melville, NY). NET percentage was calculated by dividing total number of CitH3 and SYTOX-positive NETs by the total amount of PMNs in 6 nonoverlapping fields. Experiments were repeated at least 3 times.
MPO DNA ELISA for the detection of NETs in plasma
To measure NETs in human plasma samples, a myeloperoxidase (MPO) DNA enzyme-linked immunosorbent assay (ELISA) was adapted from Caudrillier et al,26 as described in detail in supplemental Methods.
Complement ELISAs
C5a levels were determined in human plasma samples using a Human C5a ELISA kit (BMS2088, Thermo Fisher Scientific) according to the manufacturer’s instructions. The C1q-C4 complex ELISA was adapted from the report by Wouters et al.27 C1q-C4 complexes were isolated using (NH4)2SO4 precipitation. Details are provided in the supplemental Methods.
Statistical analysis
Based on normal distribution analysis, the (non)parametric statistical tests were chosen, using GraphPad Prism version 9.1.1 software for Windows (GraphPad Software, San Diego, CA), with statistical significance set at P < .05. The applied statistical tests are indicated in the figure legends, and all depicted error bars in the manuscript represent standard deviation.
Results
Antibody-mediated in vivo TRALI induction is Fc dependent and associated with in vitro complement activation
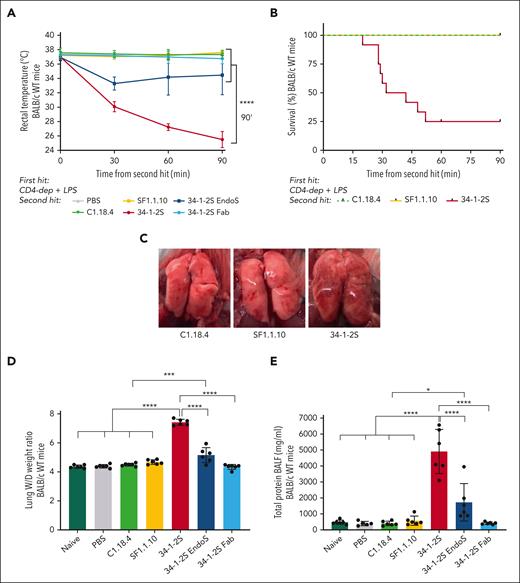
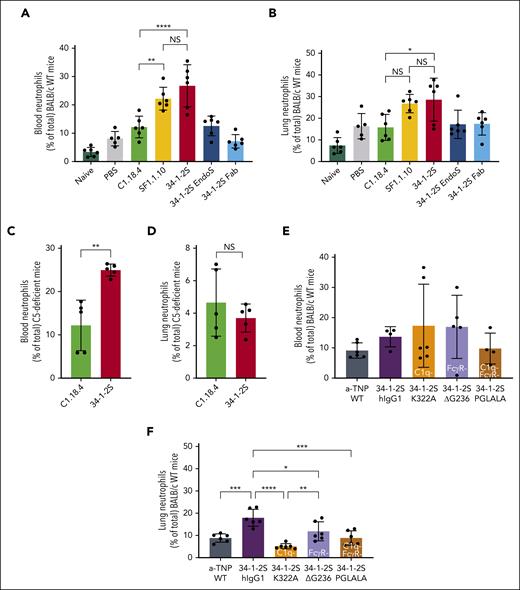
Here, we investigated the possible link between the previously reported prominent in vitro complement activation by the anti–MHC class I antibody, clone 34-1-2S,16 and its in vivo capacity to induce antibody-mediated TRALI in mice. We compared infusion of the antibody clone 34-1-2S with an anti–MHC class I antibody unable to activate complement in vitro, SF1.1.10,16 in a mouse model requiring LPS stimulation and CD4 T-cell depletion as a combined first hit to sensitize the mice.17,18 We observed that only upon infusion of 34-1-2S, a rapid drop in rectal temperatures (from 37.0°C ± 0.39°C to 25.5°C ± 1.13°C; Figure 1A) occurred. Decreased survival (25%) was observed compared with that among SF1.1.10-treated mice and corresponding control animals (100%; Figure 1B). The drop in temperature was related to the degree of acute lung injury, which was observed macroscopically (swollen and hemorrhaged lungs in the 34-1-2S group; Figure 1C, right) as well as through the determination of the lung W/D weight ratios, which corresponded to the degree of pulmonary edema (34-1-2S, 7.42 ± 0.21 vs SF1.1.10, 4.68 ± 0.16; Figure 1D) and total protein levels (mg/mL) in BALF, reflecting pulmonary endothelial permeability (Figure 1E). Infusion of 34-1-2S-Fab was unable to trigger TRALI (Figure 1D-E), indicating Fc-region requirement for induction of TRALI. Strikingly, EndoS-treated 34-1-2S, which abolished FcγR binding and reduced complement activity,16,28,29 could still induce TRALI, although diminished (Figure 1D-E). Taken together, the results from animals infused with EndoS-treated 34-1-2S (diminished complement activation and no FcγR binding) and the 34-1-2S Fab fragment (neither complement activation nor FcγR binding) and the properties of these antibodies (Table 1)16 suggest a role of the complement but not FcγR binding in inducing in vivo Fc-mediated TRALI.
In vitro antibody Fc–mediated complement activation is associated with in vivo TRALI induction. BALB/c WT mice, except for the naive group, were primed with LPS (0.1 mg/kg intraperitoneally) and CD4 T cells depleted with anti-CD4 (4.5 mg/kg; combined first hit) 16 hours before infusion of antibodies (4.5 mg/kg IV; second hit). (A) Rectal temperatures were monitored after TRALI induction. (B) Kaplan-Meier survival curve. (C) Representative images of the lungs of mice injected with C1.18.4 (left), SF1.1.10 (middle), and 34-1-2S (right). (D) Lung W/D weight ratios after 90 minutes of TRALI induction or at the time of death. (E) Protein levels in the BALF in mg/mL. Only significant differences of interest are shown; each dot represents 1 mouse; n = 5 to 6 mice per group. For panel B, 2 independent experiments were pooled; n = 11 to 12 mice per group. Data are shown as means ± standard deviation (SD). ∗P < .05; ∗∗∗P < .001; ∗∗∗∗P < .0001. Statistical analysis was performed with a one-way analysis of variance (ANOVA) with a Tukey post hoc test for panels A,D-E.
In vitro antibody Fc–mediated complement activation is associated with in vivo TRALI induction. BALB/c WT mice, except for the naive group, were primed with LPS (0.1 mg/kg intraperitoneally) and CD4 T cells depleted with anti-CD4 (4.5 mg/kg; combined first hit) 16 hours before infusion of antibodies (4.5 mg/kg IV; second hit). (A) Rectal temperatures were monitored after TRALI induction. (B) Kaplan-Meier survival curve. (C) Representative images of the lungs of mice injected with C1.18.4 (left), SF1.1.10 (middle), and 34-1-2S (right). (D) Lung W/D weight ratios after 90 minutes of TRALI induction or at the time of death. (E) Protein levels in the BALF in mg/mL. Only significant differences of interest are shown; each dot represents 1 mouse; n = 5 to 6 mice per group. For panel B, 2 independent experiments were pooled; n = 11 to 12 mice per group. Data are shown as means ± standard deviation (SD). ∗P < .05; ∗∗∗P < .001; ∗∗∗∗P < .0001. Statistical analysis was performed with a one-way analysis of variance (ANOVA) with a Tukey post hoc test for panels A,D-E.
Association of complement activation in vitro, in vivo TRALI induction, in vitro binding to FcγRs, and macrophages and PMNs in blood and lungs, all in relation to indicated antibodies
| Antibody . | Complement activation in vitro . | In vivo TRALI . | FcγR binding in vitro . | Macrophages in the blood . | Macrophages in the lungs . | PMNs in the blood . | PMNs in the lungs . |
|---|---|---|---|---|---|---|---|
| SF1.1.10 | + | – | +++ | – | ++ | +++ | +++ |
| 34-1-2S | +++ | +++ | +++ | ++ | – | +++ | +++ |
| 34-1-2S EndoS | ++ | + | – | – | + | + | + |
| 34-1-2S Fab | – | – | – | – | ++ | – | + |
| 34-1-2S hIgG1 | +++ | +++ | +++ | ++ | – | + | ++ |
| 34-1-2S K322A | – | – | +++ | + | ++ | ++ | – |
| 34-1-2S ΔG236 | +++ | +++ | – | +++ | – | ++ | ++ |
| 34-1-2S PGLALA | – | – | – | +++ | + | – | – |
| Antibody . | Complement activation in vitro . | In vivo TRALI . | FcγR binding in vitro . | Macrophages in the blood . | Macrophages in the lungs . | PMNs in the blood . | PMNs in the lungs . |
|---|---|---|---|---|---|---|---|
| SF1.1.10 | + | – | +++ | – | ++ | +++ | +++ |
| 34-1-2S | +++ | +++ | +++ | ++ | – | +++ | +++ |
| 34-1-2S EndoS | ++ | + | – | – | + | + | + |
| 34-1-2S Fab | – | – | – | – | ++ | – | + |
| 34-1-2S hIgG1 | +++ | +++ | +++ | ++ | – | + | ++ |
| 34-1-2S K322A | – | – | +++ | + | ++ | ++ | – |
| 34-1-2S ΔG236 | +++ | +++ | – | +++ | – | ++ | ++ |
| 34-1-2S PGLALA | – | – | – | +++ | + | – | – |
Complement activation in vitro and FcγR binding in vitro for antibodies SF1.1.10, 34-1-2S, 34-1-2S EndoS and 34-1-2S Fab is adapted from Zeeuw van der Laan et al.16
Complement, but not FcγRs, is critically required for in vivo antibody Fc–mediated TRALI
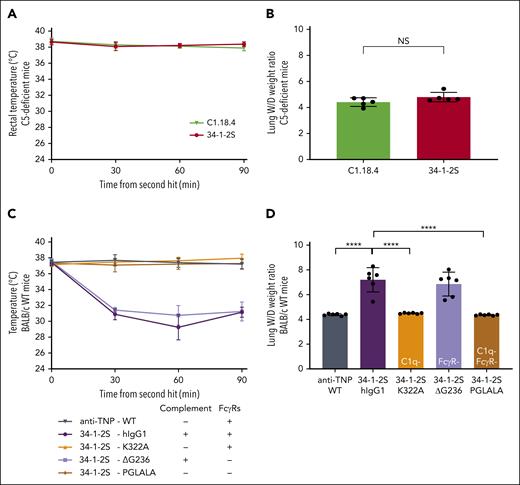
To investigate the role of complement in antibody-mediated TRALI induction, we repeated the aforementioned experiments in C5-deficient mice with antibody clone 34-1-2S and isotype control C1.18.4. Neither of the groups underwent a drop in rectal temperature (Figure 2A), nor were the lung W/D weight ratios affected (Figure 2B), indicating a C5 requirement for antibody-mediated TRALI. To exclude the role of FcγR interactions, we engineered humanized 34-1-2S IgG1 antibodies with Fc mutations, which made the antibody either unable to activate complement but with unperturbed interaction with FcγRs (34-1-2S–K322A),30 able to activate complement but unable to interact with FcγRs (34-1-2S–ΔG236),31 or unable to activate complement or interact with FcγRs (34-1-2S–PGLALA).21,22,32 All humanized 34-1-2S variants were confirmed to equally bind to H-2Kd usingsurface plasmon resonance (SPR) (supplemental Figure 2A). Their (in)capability to activate complement was confirmed in vitro using a previously established C1q-binding ELISA (supplemental Figure 2B). Furthermore, we confirmed the (in)capability of all generated 34-1-2S variants to interact with murine FcγRs (FcγRI, FcγRIIb, FcγRIII, and FcγRIV) by SPR (supplemental Figure 2C). In BALB/c WT mice, we observed that rectal temperatures dropped (Figure 2C) and lung W/D weight ratios increased (Figure 2D) only when the complement-sufficient variants of 34-1-2S (hIgG1 or ΔG236) were infused. In stark contrast, the complement-abolished but FcγR-sufficient K322A and PGLALA were unable to induce TRALI (Figure 2D). This indicated that Fc-mediated complement activation, but not Fc interaction with FcγRs, is essential for the development of antibody-mediated TRALI.
Complement, in contrast to FcγRs, is essential for in vivo antibody-mediated TRALI induction. C5-deficient (A-B) and BALB/c WT mice (C-D) were primed as described in Figure 1. (A,C) Rectal temperatures and (B,D) lung W/D weight ratios after 90 minutes of antibody infusion (C5-deficient mice: 4.5 mg/kg IV; BALB/c WT mice: 1 mg/kg IV). All human antibodies (C-D) were hIgG1 chimeric variants. Variants used were K322A (no C1q binding), ΔG236 (no FcγR binding), and PGLALA (no C1q/FcγR binding). Only statistical differences of interest are shown; each dot represents 1 mouse. n = 6; rectal temperatures: n = 2 for 34-1-2S–hIgG1; n = 5 for 34-1-2S and for 34-1-2S–ΔG236 because of reaching humane end points; other groups, n = 6. Data are shown as means ± SD; ∗∗∗∗P < .0001. Statistical analysis was performed with a one-way ANOVA with a Tukey post hoc test. NS, nonsignificant.
Complement, in contrast to FcγRs, is essential for in vivo antibody-mediated TRALI induction. C5-deficient (A-B) and BALB/c WT mice (C-D) were primed as described in Figure 1. (A,C) Rectal temperatures and (B,D) lung W/D weight ratios after 90 minutes of antibody infusion (C5-deficient mice: 4.5 mg/kg IV; BALB/c WT mice: 1 mg/kg IV). All human antibodies (C-D) were hIgG1 chimeric variants. Variants used were K322A (no C1q binding), ΔG236 (no FcγR binding), and PGLALA (no C1q/FcγR binding). Only statistical differences of interest are shown; each dot represents 1 mouse. n = 6; rectal temperatures: n = 2 for 34-1-2S–hIgG1; n = 5 for 34-1-2S and for 34-1-2S–ΔG236 because of reaching humane end points; other groups, n = 6. Data are shown as means ± SD; ∗∗∗∗P < .0001. Statistical analysis was performed with a one-way ANOVA with a Tukey post hoc test. NS, nonsignificant.
Antibody-mediated and Fc-induced complement-dependent TRALI is associated with macrophage trafficking from the lungs to the blood
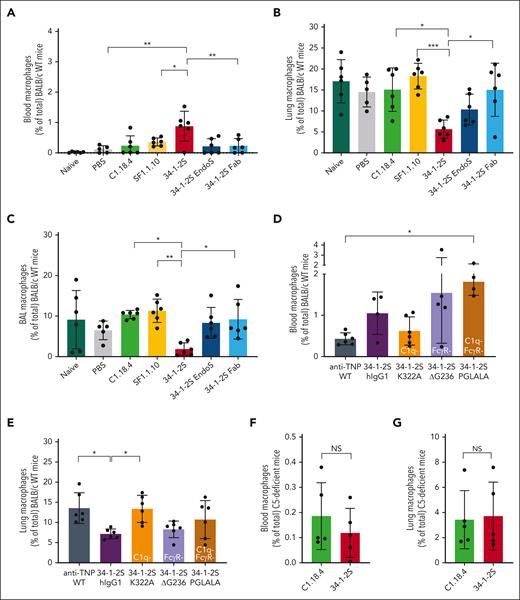
Previously, it has been shown that macrophages play a major role in TRALI pathogenicity, because in vivo depletion of macrophages protected mice from TRALI.19,33 To examine whether macrophages could be associated with complement activation, we measured macrophage numbers in the blood, lung tissue, and BALF in these TRALI models. We observed an increase in macrophage levels in the blood of 34-1-2S TRALI mice compared with that in infused mice with complement activation lacking SF1.1.10 and in control animals (Figure 3A). Macrophage levels were decreased in the lung tissue and BALF of 34-1-2S TRALI mice compared with in SF1.1.10–infused animals (Figure 3B-C). We showed that this was Fc mediated, because observations with 34-1-2S–Fab or EndoS-treated 34-1-2S were similar to those in the control groups (Figure 3A-C). Treatment with 34-1-2S–ΔG236, which was unable to activate FcγRs, showed similar characteristics as 34-1-2S–hIgG1 (Figure 3D-E), suggesting a nonessential role for FcγRs in relation to TRALI induction and (CD11b−) macrophage trafficking (supplemental Figure 3A-B). In complement-disabled 34-1-2S–K322A (Figure 3E) and C5-deficient mice (Figure 3F-G), we observed similar macrophage levels as in their respective controls in both the blood and lungs. Collectively, this indicates that macrophages traffic from the lungs to the blood during complement-dependent antibody-mediated TRALI.
Fc-mediated complement activation is associated with macrophage trafficking from the lungs to the blood in in vivo antibody-mediated TRALI. BALB/c WT (A-E) and C5-deficient (F-G) mice were primed as in Figures 1 and 2, followed by infusion of indicated antibodies. (A) Blood, (B) lung tissue, and (C) BALF of BALB/c WT mice treated with respective antibodies 4.5 mg/kg IV, and (D) blood and (E) lung tissue of BALB/c WT mice treated with the indicated human chimeric antibodies, 1 mg/kg IV. (F) Blood and (G) lung tissue of C5-deficient mice (C57BL/6 with H2-kd) treated with mouse IgG2a antibodies (4.5 mg/kg IV). Only statistical differences of interest are shown; each dot represents 1 mouse. Data are shown as means ± SD; n = 4 to 6; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. Statistical analysis was performed with a one-way ANOVA with a Tukey post hoc test.
Fc-mediated complement activation is associated with macrophage trafficking from the lungs to the blood in in vivo antibody-mediated TRALI. BALB/c WT (A-E) and C5-deficient (F-G) mice were primed as in Figures 1 and 2, followed by infusion of indicated antibodies. (A) Blood, (B) lung tissue, and (C) BALF of BALB/c WT mice treated with respective antibodies 4.5 mg/kg IV, and (D) blood and (E) lung tissue of BALB/c WT mice treated with the indicated human chimeric antibodies, 1 mg/kg IV. (F) Blood and (G) lung tissue of C5-deficient mice (C57BL/6 with H2-kd) treated with mouse IgG2a antibodies (4.5 mg/kg IV). Only statistical differences of interest are shown; each dot represents 1 mouse. Data are shown as means ± SD; n = 4 to 6; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. Statistical analysis was performed with a one-way ANOVA with a Tukey post hoc test.
Murine TRALI is associated with C5a chemoattraction of neutrophils and complement-dependent NETosis
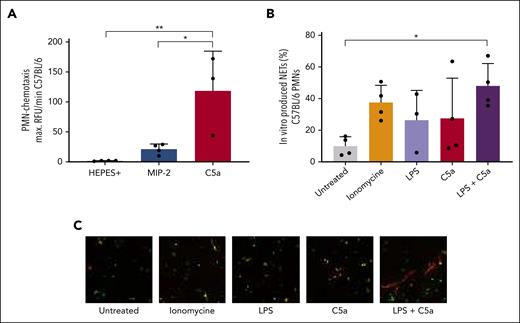
We have previously shown that PMN lung infiltration is required for TRALI induction.18 To investigate whether PMNs would demonstrate similar complement activation–dependent trafficking as observed for macrophages, we determined PMN numbers in the blood and lungs of 34-1-2S–TRALI mice. Upon comparing 34-1-2S with isotype antibody C1.18.4 infusion, we observed an increase of PMNs in the blood (Figure 4A) and lungs (Figure 4B). The increase of PMN numbers in the blood (Figure 4C) but not in the lungs (Figure 4D) was also seen in 34-1-2S–treated C5-deficient mice. In BALB/c mice induced with the chimeric 34-1-2S, we again observed a complement-dependent influx of PMNs in the lungs (Figure 4F), which was in line with the well-established role for C5a as a neutrophil chemoattractant.34 Compared with controls, C5a acted as a potent PMN chemoattractant in a PMN chemotaxis assay (Figure 5A). Together, this suggested that C5a may contribute to PMN attraction to the lungs in TRALI. Administration of chimeric antibody variants of clone 34-1-2S did not alter blood PMN levels (Figure 4E), suggesting that other mechanisms are responsible for PMN attraction to the blood than to the lungs. In addition, we did not observe a difference in PMN numbers between the groups treated with the TRALI-inducing 34-1-2S antibody and those treated with the SF1.1.10 antibody (Figure 4A-B), which has diminished complement activation abilities in vitro and that was neither able to cause TRALI in vivo (Figure 1B) nor affect the number of blood macrophages (Figure 3A). We hypothesized that PMN measurements may have been compromised because of NET formation in the 34-1-2S–treated animals. We, therefore, investigated the ability of PMNs to form NETs by mimicking the 2-hit murine TRALI reaction in vitro with LPS (first hit) and recombinant C5a (imitating complement activation due to the second hit). Using wide-field microscopy–based quantification of extracellular DNA and citrullinated histone H3 staining, we observed that NETs were formed more abundantly in this 2-hit model than in single-stimulation settings (Figure 5B-C), indicating that complement can trigger NET formation in a 2-event in vitro setting.
Neutrophil levels are increased in the blood and lungs of WT TRALI mice but not in lungs of C5-deficient mice. Neutrophils were assessed in the blood (A,C,E) and lung tissue (B,D,F) in BALB/c WT (A-B,E-F) mice or C5-deficient mice (C), treated as in Figures 1-3 and infused with 4.5 mg/kg of specified antibodies IV or (E-F) 1 mg/kg IV for the human chimeric antibody variants. Only statistical differences of interest are shown; each dot represents 1 mouse; n = 5 to 6. Data are shown as means ± SD. Statistical analysis was performed with a one-way ANOVA with a Tukey post hoc test; ∗P <.05; ∗∗P < .01; ∗∗∗∗P < .0001.
Neutrophil levels are increased in the blood and lungs of WT TRALI mice but not in lungs of C5-deficient mice. Neutrophils were assessed in the blood (A,C,E) and lung tissue (B,D,F) in BALB/c WT (A-B,E-F) mice or C5-deficient mice (C), treated as in Figures 1-3 and infused with 4.5 mg/kg of specified antibodies IV or (E-F) 1 mg/kg IV for the human chimeric antibody variants. Only statistical differences of interest are shown; each dot represents 1 mouse; n = 5 to 6. Data are shown as means ± SD. Statistical analysis was performed with a one-way ANOVA with a Tukey post hoc test; ∗P <.05; ∗∗P < .01; ∗∗∗∗P < .0001.
NETs are induced in an in vitro TRALI setting with C5a, which mimics the complement-inducing effect of 34-1-2S. (A) Neutrophils from the bone marrow of C57BL/6 WT mice in a chemotaxis assay under indicated conditions. (B) In vitro NET formation under indicated conditions. (C) Representative images; original magnification ×20, with digital zoom to increase visibility. Only statistical differences of interest are shown; each dot represents neutrophils isolated from 1 individual mouse, n = 3 to 4. Data are shown as means ± SD. Statistical analysis was performed with a one-way ANOVA with a Tukey post hoc test; ∗P < .05; ∗∗P <.01.
NETs are induced in an in vitro TRALI setting with C5a, which mimics the complement-inducing effect of 34-1-2S. (A) Neutrophils from the bone marrow of C57BL/6 WT mice in a chemotaxis assay under indicated conditions. (B) In vitro NET formation under indicated conditions. (C) Representative images; original magnification ×20, with digital zoom to increase visibility. Only statistical differences of interest are shown; each dot represents neutrophils isolated from 1 individual mouse, n = 3 to 4. Data are shown as means ± SD. Statistical analysis was performed with a one-way ANOVA with a Tukey post hoc test; ∗P < .05; ∗∗P <.01.
Blockade of the C5a-receptor (C5aR) does not prevent development of antibody-mediated murine TRALI
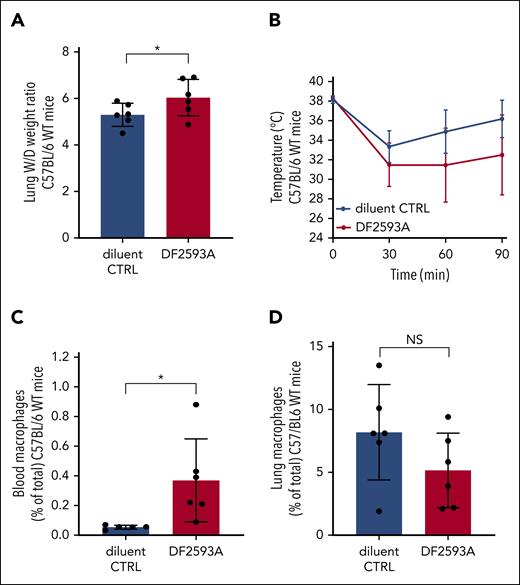
Considering the apparent critical importance of complement in driving antibody-mediated murine TRALI, we investigated whether blockade of the C5aR, via the noncompetitive and allosteric small chemical antagonist of C5aR, DF2593A,35 could prevent TRALI development. For this, we used our previously established antibody-mediated TRALI model in C57BL/6 mice (supplemental Figure 4),19 based on applying a combined first hit with LPS and CD4 T-cell depletion, followed by infusion of a high dose of 34-1-2S, which binds to H-2Kd with slight cross-reactivity to H-2Kb, combined with AF6-88.5.5.3, which strongly binds to H-2Kb.19 Infusion of DF2593A did not prevent the development of TRALI. In contrast, TRALI outcome worsened, as was evident from increased lung W/D weight ratios and a slight, although not significant, decrease in rectal temperature when DF2593A was injected compared with the diluent control together with 34-1-2S and AF6-88.5.5.3 (Figure 6A-B). We observed that this adverse effect was related to the increment of blood macrophage levels (Figure 6C) and a trend in decreased lung tissue macrophage numbers (Figure 6D).
Blocking the C5aR does not prevent antibody-mediated murine TRALI. C57BL/6 WT mice were primed as described earlier (Figure 1), followed by IV infusion of both 34-1-2S (45 mg/kg) and AF6-88.5.5.3 (4.5 mg/kg) together with diluent control or the small chemical C5aR antagonist, DF2593A (10 mg/kg). (A) Lung W/D weight ratio at 90 minutes; (B) rectal temperatures; and (C) macrophage levels in the blood and (D) lung at 90 minutes, n = 6. All dots represent individual mice. All mice survived until the 90-minute time point. Data are shown as means ± SD. Statistical analyses was performed with a 1-tailed unpaired t test; ∗P < .05. CTRL, control.
Blocking the C5aR does not prevent antibody-mediated murine TRALI. C57BL/6 WT mice were primed as described earlier (Figure 1), followed by IV infusion of both 34-1-2S (45 mg/kg) and AF6-88.5.5.3 (4.5 mg/kg) together with diluent control or the small chemical C5aR antagonist, DF2593A (10 mg/kg). (A) Lung W/D weight ratio at 90 minutes; (B) rectal temperatures; and (C) macrophage levels in the blood and (D) lung at 90 minutes, n = 6. All dots represent individual mice. All mice survived until the 90-minute time point. Data are shown as means ± SD. Statistical analyses was performed with a 1-tailed unpaired t test; ∗P < .05. CTRL, control.
Complement activation markers and NET levels positively correlate with each other in the plasma of patients with TRALI
To investigate the relevance and association of complement and NETs in human TRALI, we assessed the presence of C5a, C1q-C4 complexes, and NET markers in plasma samples of patients with TRALI (n = 53), of which we assessed interleukin-6 (IL-6), IL-8, IL-10, and C-reactive protein (CRP) levels. We found that IL-10 levels were not altered (supplemental Figure 5A) and IL-6, IL-8, and CRP were significantly elevated (supplemental Figure 5B,C,D, respectively), compared with in healthy controls. We found high levels of C5a (Figure 7A), C1q-C4 complexes (Figure 7B), and NETs (Figure 7C) in patients with TRALI. We observed an association between elevated C5a levels and NET formation (n = 53; ρ = 0.294; Figure 7D). We did not observe this association in healthy controls (Figure 7E), supporting the importance of complement and NET association in patients with TRALI, indicating the need to explore the targeting of the complement system as a therapeutic for combating TRALI.
Complement and NETs are increased and correlated with each other in plasma samples from human patients with TRALI. (A) C5a levels (patient with TRALI [TP]: n = 53; healthy control [HC]: n = 30), (B) C1q-C4 complex levels (TP: n = 46; HC: n = 25), and (C) NET formation (MPO DNA; TP: n = 53; HC: n = 30). C5a and NET levels are positively correlated to each other in (D) TPs (n = 53) but not in (E) HCs (n = 30). Data are shown as means ± SD. Statistical analyses were performed with a Mann Whitney U test for panels A-C and a Pearson correlation for panels D-E; ∗P < .05; ∗∗∗P < .001. OD, optical density.
Complement and NETs are increased and correlated with each other in plasma samples from human patients with TRALI. (A) C5a levels (patient with TRALI [TP]: n = 53; healthy control [HC]: n = 30), (B) C1q-C4 complex levels (TP: n = 46; HC: n = 25), and (C) NET formation (MPO DNA; TP: n = 53; HC: n = 30). C5a and NET levels are positively correlated to each other in (D) TPs (n = 53) but not in (E) HCs (n = 30). Data are shown as means ± SD. Statistical analyses were performed with a Mann Whitney U test for panels A-C and a Pearson correlation for panels D-E; ∗P < .05; ∗∗∗P < .001. OD, optical density.
Discussion
The complement system is well known for its important role in the host defense and surveillance system but also as an initiator, contributor, and/or exacerbator in various disorders.36,37 We previously investigated the differences between a TRALI-inducing and a non–TRALI-inducing antibody from a biological and structural perspective in vitro.16 In this study, we have linked the previously found in vitro results16 with in vivo antibody-mediated TRALI induction. Although complement has previously been linked to TRALI, studies investigating complement involvement in TRALI have been remarkably limited and have presented inconsistent outcomes.14 Here, we showed that in vivo antibody-mediated TRALI is correlated with the in vitro complement-activation strength of the tested antibodies and that this was fully Fc mediated because the infusion of 34-1-2S Fab was unable to induce TRALI. Next, we investigated whether TRALI induction was critically dependent on complement activation or via interaction with FcγRs. Discrepancies were found between previous studies regarding FcγR involvement in TRALI.15,38 Looney et al have previously showed that FcγR-deficient BALB/c mice failed to develop antibody-mediated TRALI.38 In contrast, Strait et al showed that murine TRALI can be suppressed by activating the inhibiting FcγRIIb but that inhibition of the other FcγRs (FcγRI, FcγRIII, and FcγRIV) did not affect TRALI outcomes.15,39 This observation was also made by Bayat et al, who suggested that, in mice infused with human anti-HNA-3a, FcγR-dependent neutrophil attraction to the lungs can worsen the murine TRALI outcome.40 These conflicting results may be due to differences in experimental conditions, readouts for acute lung injury, or animal housing conditions.14,15 We, therefore, generated humanized IgG1 variants of 34-1-2S containing mutations rendering the 34-1-2S incapable of activating complement and/or of interacting with murine FcγRs. These experiments strongly suggested that Fc-mediated complement activation, but not Fc interaction with FcγRs, was essential for development of antibody-mediated murine TRALI. In accordance with this outcome and with previous experiments by Strait et al,15 we observed that C5-deficient mice did not develop antibody-mediated TRALI.15
In relation to the antibody Fc–mediated and complement-driven TRALI reaction, we analyzed the number of macrophages and PMNs, known pathogenic cells in TRALI,6,12 in the blood and in lung tissue of TRALI mice. We have previously shown that macrophage depletion protected mice from antibody-mediated TRALI.19 Furthermore, macrophages have been associated with murine TRALI, because they have been linked to C5a binding,15 which acts as a strong chemoattractant for macrophages via the release of reactive oxygen species and inflammatory cytokines.41 Macrophages are extremely diverse in function and phenotype, and the alveolar macrophages are especially important for maintaining homeostasis in the lungs.33 In murine TRALI pathogenesis, macrophages are polarized toward the proinflammatory M1-state33 with increased proinflammatory cytokine secretion, such as IL-6 and IL-8.33 Intriguingly, we observed that during the onset of antibody Fc–mediated and complement-dependent murine TRALI, macrophages appeared to traffic from the lungs to the blood. In the animals infused with 34-1-2S–PGLALA, we demonstrate an unexplained increase of blood macrophages. Comparing the 34-1-2S–WT and its Fc-receptor effector function–deficient but complement-sufficient variants in the TRALI groups demonstrated that it is the combination of increased macrophage levels in the blood and decreased macrophage levels in the lungs that is important. CRP can enhance IL-8 secretion,42 and we hypothesize that this contributes to the removal of the CD11b− lung tissue–resident macrophages, by attracting them to the blood, which can convey protective tissue-specific functions in relation to maintaining homeostasis; this needs to be further studied in detail.43 During this reaction, the anti-inflammatory resident macrophages may egress from the lungs, which could lower the threshold for induction of lung endothelium damage in TRALI. In our human plasma TRALI cohort, we showed increased levels of IL-6 and IL-8, whereas IL-10 levels remained low.
Previously, it has been shown that depletion of PMNs prevented the occurrence of antibody-mediated murine TRALI,17,18 and the importance of PMNs was again underlined in this study in which we observed an increase of PMNs in the lung tissue of TRALI mice. In line with these findings, we did not observe an increase of PMNs in the lungs of C5-deficient mice or in mice treated with the complement-disabled hIgG1 variants 34-1-2S–K322A and 34-1-2S–PGLALA, which did not develop TRALI. These data showed an important, pathogenic, chemoattractant role of C5a in the lungs during TRALI. Surprisingly, we observed that PMNs were similarly increased in mice infused with the TRALI-inducing antibody 34-1-2S, compared with the non–TRALI-inducing antibody SF1.1.10. This might be because of a secondary FcγR-mediated activation method that is not complement dependent because we also observed an increase of PMNs in the blood of C5-deficient mice infused with 34-1-2S. We subsequently hypothesized that complement-induced NETosis in 34-1-2S–TRALI mice results in reduced PMN counts compared with that in SF1.1.10-infused mice, because PMNs that underwent NETosis are likely to be missed.44 This is supported by previous statements about NETs to be present in TRALI mice,26,45 and Caudrillier et al effectively show that administration of DNase 1 protected mice from TRALI induction.26 In vitro, we showed that C5a functions as a potent PMN chemoattractant, and in conjunction with the first hit of our murine TRALI model, LPS triggers the formation of NETs. In plasma samples from 53 patients with TRALI, to our knowledge the largest tested human TRALI cohort to date, for analyses of complement and NETs, we measured high levels of C5a and abundant NET formation, which positively correlated with each other. Although there are still some differences in readouts for NETs in plasma samples, these findings are in line with an earlier study of Ortiz-Espinosa et al in which they also demonstrate a correlation between C5a and increased MPO DNA levels.46 This supports that C5a might have been a trigger for NET formation in TRALI. Further studies should investigate the mechanistic pathways interlinking the complement system and NETs in TRALI.
TRALI is a leading cause of blood transfusion-related fatalities,1,3-6 with no specific therapies available.2 Because of the identified essential role for complement in driving antibody-mediated murine TRALI, we tested the potential of preventing TRALI by blocking the C5aR in vivo using a selective, noncompetitive, allosteric antagonist of C5aR, DF2593A. Despite the previous results from Strait et al,15 in which they showed that C5aR-deficient mice were unable to develop TRALI, and those of Looney et al who demonstrated a normal disease development in C5aR-deficient mice,38 we observed an unexpected worsening of the TRALI reaction and increased blood macrophage numbers upon infusion of the C5aR-antagonist DF2593A. Although this further underlined the association of TRALI severity with a disturbance of macrophage and neutrophil distribution, it also pointed toward an important relationship between location and abundance of cells as a cohesive threshold for TRALI induction. The adverse effect of blocking C5aR, whereas C5 deficiency was protective, suggests a complex role of complement in TRALI. Possibly, recruitment of immune cells via the C5a–C5aR axis prevents TRALI exacerbation by removal of immune complexes, dead cells, or cell debris. Simultaneously, generation of C5b, and subsequent formation of the lytic membrane attack complex may drive TRALI. We are currently investigating whether preventing C5b and lytic membrane attack complex formation is more beneficial in our TRALI model.
In conclusion, we used previously characterized antibody variants differing in potency to activate complement in vitro and assessed their TRALI-inducing ability in vivo. We used C5-deficient mice to investigate the requirement for complement activation, and we engineered the TRALI-inducing antibody 34-1-2S with mutations that silence complement and/or FcγR effector functions, which convincingly revealed the critical requirement for complement but not for FcγRs in TRALI induction. We could link this complement activity to macrophage trafficking from the lungs to the blood, and to in vitro NET formation. In support, we demonstrated increased levels of complement and NETs in a large cohort of patients with TRALI, compared with healthy controls. We were not able to acquire samples from control patients who had undergone transfusion, and we are aware that cytokine levels can be elevated as a response to a transfusion alone,47 but we believe that the positive association of complement and NETs in our human TRALI cohort, together with the extensive murine TRALI data, strongly support the critical role of complement in TRALI. TRALI pathogenicity may be initiated by antibody binding to MHC I on the pulmonary endothelium, resulting in increased antibody Fc–mediated C5a generation through the classical pathway of complement activation, which consequently stimulates trafficking of protective homeostatic alveolar macrophages from the lungs to the blood. C5a generation can cause direct damage to the endothelial cells, and, in addition, it attracts PMNs that could, under the influence of enhanced C5a levels, undergo NETosis. These formed NETs would then be able to contribute to the endothelium damage, facilitating PMN transendothelial migration into the lungs. Development of pulmonary edema and TRALI is critically dependent on complement–mediated macrophage trafficking and, as our current findings suggest, NET formation, but this needs to be further investigated. Our data indicate that, unlike blocking the C5aRs, targeting C5 directly may be a promising therapeutic strategy to explore in combating classical antibody-mediated TRALI.
Acknowledgment
This work was supported by Sanquin (grant PPOC-18-08).
Authorship
Contribution: S.v.d.V., T.L.J.v.O., A.S., and A.E.H.B. performed experiments and analyzed data; R.K. analyzed data and supervised the study; S.v.d.V. and R.K. designed the experiments and wrote the manuscript; and all authors critically reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rick Kapur, Department of Experimental Immunohematology, Sanquin Research and Landsteiner Laboratory, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; email: r.kapur@sanquin.nl.
References
Author notes
Data were presented in oral abstract form at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 12 December 2021.
Data are available on request from the corresponding author, Rick Kapur (r.kapur@sanquin.nl).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Complement and NETs are increased and correlated with each other in plasma samples from human patients with TRALI. (A) C5a levels (patient with TRALI [TP]: n = 53; healthy control [HC]: n = 30), (B) C1q-C4 complex levels (TP: n = 46; HC: n = 25), and (C) NET formation (MPO DNA; TP: n = 53; HC: n = 30). C5a and NET levels are positively correlated to each other in (D) TPs (n = 53) but not in (E) HCs (n = 30). Data are shown as means ± SD. Statistical analyses were performed with a Mann Whitney U test for panels A-C and a Pearson correlation for panels D-E; ∗P < .05; ∗∗∗P < .001. OD, optical density.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/1/10.1182_blood.2023020484/4/m_blood_bld-2023-020484-gr7.jpeg?Expires=1765883867&Signature=cI7enaS7rXDivKpfBNIIsUApDNryun5ED~9Ts3k4IjHCLtdTyTDCJ91AVwAmhd9Nrtw8Y3I9P4Nlim65J4sRoZAK4~qjA0Gu9FC7iav8bbfmejQhbwpMlso~4x3-BRiOZ9j3hy8XEsq7aQnHLRRygSXdiQ1hGC1MTZsoK7bKVo4b7R1JZLMZPH4exGck58FOgKQPP~Y4AeIc2PiUJGg2K3vCOXHNFYwADmzc6R~2jPwuQvej8K036Xf3hLBwGgezyjqEi6JBh95GfXrrBt6cjdb6lb1NUon~Q8uyxFY7BrVke-eKgOEX7je0UtTd2OjlwQmQhnUDGvaoCb2bV6mn-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal