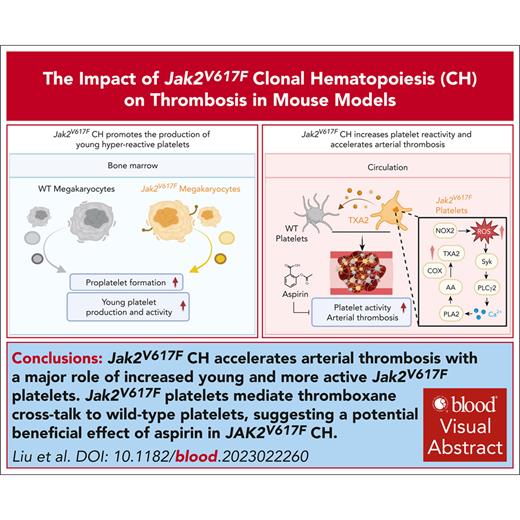
Jak2VF CH accelerated arterial thrombosis with a major role of increased young and more active Jak2VF platelets.
Jak2VF platelets mediate thromboxane cross talk to WT platelets, suggesting a potentially beneficial effect of aspirin in JAK2VF CH.
Visual Abstract
JAK2V617F (JAK2VF) clonal hematopoiesis (CH) has been associated with atherothrombotic cardiovascular disease (CVD). We assessed the impact of Jak2VF CH on arterial thrombosis and explored the underlying mechanisms. A meta-analysis of 3 large cohort studies confirmed the association of JAK2VF with CVD and with platelet counts and adjusted mean platelet volume (MPV). In mice, 20% or 1.5% Jak2VF CH accelerated arterial thrombosis and increased platelet activation. Megakaryocytes in Jak2VF CH showed elevated proplatelet formation and release, increasing prothrombogenic reticulated platelet counts. Gp1ba-Cre–mediated expression of Jak2VF in platelets (VFGp1ba) increased platelet counts to a similar level as in 20% Jak2VF CH mice while having no effect on leukocyte counts. Like Jak2VF CH mice, VFGp1ba mice showed enhanced platelet activation and accelerated arterial thrombosis. In Jak2VF CH, both Jak2VF and wild-type (WT) platelets showed increased activation, suggesting cross talk between mutant and WT platelets. Jak2VF platelets showed twofold to threefold upregulation of COX-1 and COX-2, particularly in young platelets, with elevated cPLA2 activation and thromboxane A2 production. Compared with controls, conditioned media from activated Jak2VF platelets induced greater activation of WT platelets that was reversed by a thromboxane receptor antagonist. Low-dose aspirin ameliorated carotid artery thrombosis in VFGp1ba and Jak2VF CH mice but not in WT control mice. This study shows accelerated arterial thrombosis and platelet activation in Jak2VF CH with a major role of increased reticulated Jak2VF platelets, which mediate thromboxane cross talk with WT platelets and suggests a potential beneficial effect of aspirin in JAK2VF CH.
Introduction
JAK2V617F (JAK2VF) is the most common somatic mutation that drives development of myeloproliferative neoplasms (MPNs) with markedly increased atherothrombotic risk.1JAK2VF can also give rise to clonal hematopoiesis (CH), a highly prevalent condition in older adults as a result of somatic mutations that provide a fitness advantage to hematopoietic stem and progenitor cells leading to clonal expansion of mutant leukocytes in blood. When this occurs in the absence of clinical manifestations of MPN and at a variable allele frequency (VAF) > 2%, it is known as clonal hematopoiesis of indeterminate potential (CHIP). Several studies have shown that CH is an independent risk factor for atherothrombotic cardiovascular disease (CVD).2-5 Among the common genetic variants underlying CH, JAK2VF markedly increases risk of CVD, including premature myocardial infarction.2 In addition, JAK2VF CH increases venous thrombotic risk and venous thromboembolism.6 Despite the adverse impact of JAK2VF on CVD, there are no approved therapies to reduce this risk specifically in CH.
JAK2VF is a gain-of-function variant that increases hematopoietic cytokine signaling in multiple blood cell lineages. Recently, we demonstrated increased atherosclerosis and necrotic core formation in mice expressing Jak2VF selectively in macrophages and in chimeric mice that model CH.7 We also showed that selective expression of Jak2VF in erythrocytes promotes atherosclerosis via increased erythrophagocytosis and ferroptosis of atherosclerotic lesional macrophages.8 Moreover, Jak2VF CH exacerbated pathological remodeling in murine heart failure.9
Despite these advances, the impact of Jak2VF CH on thrombosis has not been assessed in animal models and the causality is not known. Here, we report that mouse models simulating human JAK2VF CH display increased platelet production and reactivity and accelerated arterial thrombosis, involving the generation of young hyperreactive platelets and prothrombotic cross talk between mutant and WT platelets.
Methods
Mice
Wild-type (WT; 000664), green fluorescent protein–positive (GFP+; 006567), and Ldlr−/− (002207) mice were purchased from The Jackson Laboratory. Jak2V617F mice were created previously.10 EpoR-Cre mice were originally generated by Achim Heinrich et al,11 and Gp1ba-Cre mice were originally generated by Yotis A. Senis et al.12 All mice were housed under 12-hour light-dark cycles and expanded in a pathogen-free condition.
Thrombosis assays
For FeCl3-injury thrombosis assay, mice were anesthetized with isoflurane and the carotid artery was exposed. A filter paper soaked in 10% FeCl3 was applied to the artery for 3 minutes, and then blood flow was monitored with an ultrasound flow probe (Transonics). The time to total occlusion was defined as from the time point when FeCl3 filter paper was applied to a complete occlusion of the artery with zero blood flow. After the procedure, all mice were euthanized immediately.
Detailed methods and the statistical analysis are described in supplemental Material, available on the Blood website.
Results
Effect of JAK2V617F CH on CAD
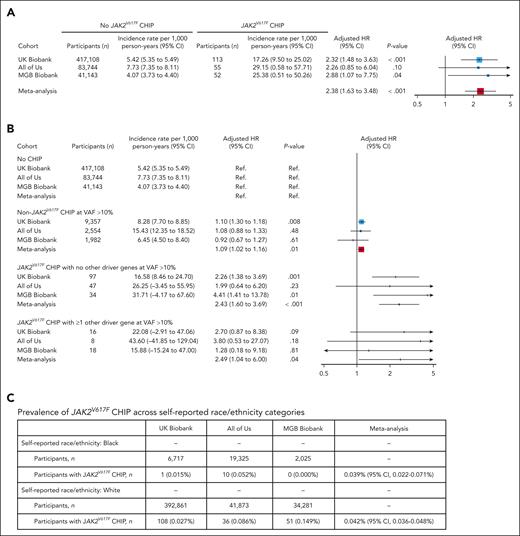
We carried out a meta-analysis of the effects of JAK2VF CHIP on coronary artery disease (CAD) events in UK Biobank, All of Us, and MGB Biobank cohorts. Consistent with previous findings,2 meta-analysis showed a 2.4-fold increase in incident CAD events in association with JAK2VF CHIP, with comparable increases across the individual cohorts (Figure 1A; supplemental Figures 1 and 2). We compared CAD risk in association with CHIP driven exclusively by JAK2VF CHIP, non-JAK2VF CHIP, or JAK2VF CHIP with additional CHIP mutations. CHIP driven by JAK2VF was associated with a 2.4-fold increase in CAD events (P < .001). This risk was increased 2.5-fold when combined with additional CHIP mutations (Figure 1B). Non-JAK2VF CHIP was associated with a 1.09-fold increase in CAD events (P < .01; Figure 1B). When stratified based on hemoglobin level and platelet and neutrophil counts <1 standard deviation (SD) vs >1 SD from the mean of all JAK2V617F CHIP carriers, the hazard ratio (HR) of incident CAD in UK Biobank for participants within 1 SD (n = 44) vs noncarriers was 2.18 (95% confidence interval [CI], 1.04-4.57; P = .039), and the HR for participants with SD >1 (n = 64) vs noncarriers was 2.06 (95% CI, 1.11-3.82; P = .023). JAK2VF was detected in all racial groups. Unadjusted (Fisher exact) or adjusted (logistic regression models adjusted for age, square of age, sex, smoking status, and body mass index) tests revealed no statistically significant association of race (Black vs White), with prevalence of JAK2VF CHIP for the individual cohorts or in meta-analysis (Figure 1C), albeit with prominence of White participants in UK Biobank and MGB Biobank. Fixed-effects meta-analysis of the effect of race (Black vs White) on JAK2VF CHIP prevalence yielded a meta-analyzed multivariable-adjusted odds ratio (OR) of 0.88 (95% CI, 0.43-1.81; P = .73), indicating JAK2VF CHIP is detected in both Black and White subjects.
Effect of JAK2V617F (JAK2VF) CHIP on CAD. Meta-analysis of the effects of JAK2VF CHIP on the occurrence of CAD events in All of Us, UK Biobank, and MGB Biobank. (A) Multivariable-adjusted logistic regression model including age, square of age, sex, self-reported race, principal components of ancestry 1 to 10, smoking status, body mass index (BMI), systolic blood pressure, antihypertensive medication use, hyperlipidemia, cholesterol-lowering medication use, and prevalent diabetes mellitus as covariates. (B) Association of JAK2VF CHIP with CAD in the presence or absence of non-JAK2VF CHIP clones. Participants were categorized in 4 groups based on the presence of (1) JAK2VF CHIP mutations and (2) large non-JAK2VF CHIP mutations (ie, VAF > 10%). This figure shows the associations of each of these groups with incident CAD events in the UK Biobank, All of Us, and MGB Biobank. Effects were estimated using Cox proportional hazards models including age, square of age, sex, self-reported race, principal components of ancestry 1 to 10, smoking status, BMI, systolic blood pressure, antihypertensive medication use, hyperlipidemia, cholesterol-lowering medication use, and prevalent diabetes mellitus as covariates. Participants without CHIP (no CHIP) serving as the reference group (Ref). (C) Fixed-effects meta-analysis of the proportion of JAK2VF CHIP across study cohorts yielded comparable estimates for participants who self-reported as White vs Black. No unadjusted (Fisher exact) or adjusted (logistic regression models adjusted for age, square of age, sex, smoking status, and BMI) tests revealed a statistically significant association of race (Black vs White) with prevalence of JAK2VF CHIP for the individual cohorts. Fixed-effects meta-analysis of the effect of race (Black vs White) on JAK2VF CHIP prevalence yielded a meta-analyzed multivariable-adjusted OR of 0.88 (95% CI, 0.43-1.81; P = .73).
Effect of JAK2V617F (JAK2VF) CHIP on CAD. Meta-analysis of the effects of JAK2VF CHIP on the occurrence of CAD events in All of Us, UK Biobank, and MGB Biobank. (A) Multivariable-adjusted logistic regression model including age, square of age, sex, self-reported race, principal components of ancestry 1 to 10, smoking status, body mass index (BMI), systolic blood pressure, antihypertensive medication use, hyperlipidemia, cholesterol-lowering medication use, and prevalent diabetes mellitus as covariates. (B) Association of JAK2VF CHIP with CAD in the presence or absence of non-JAK2VF CHIP clones. Participants were categorized in 4 groups based on the presence of (1) JAK2VF CHIP mutations and (2) large non-JAK2VF CHIP mutations (ie, VAF > 10%). This figure shows the associations of each of these groups with incident CAD events in the UK Biobank, All of Us, and MGB Biobank. Effects were estimated using Cox proportional hazards models including age, square of age, sex, self-reported race, principal components of ancestry 1 to 10, smoking status, BMI, systolic blood pressure, antihypertensive medication use, hyperlipidemia, cholesterol-lowering medication use, and prevalent diabetes mellitus as covariates. Participants without CHIP (no CHIP) serving as the reference group (Ref). (C) Fixed-effects meta-analysis of the proportion of JAK2VF CHIP across study cohorts yielded comparable estimates for participants who self-reported as White vs Black. No unadjusted (Fisher exact) or adjusted (logistic regression models adjusted for age, square of age, sex, smoking status, and BMI) tests revealed a statistically significant association of race (Black vs White) with prevalence of JAK2VF CHIP for the individual cohorts. Fixed-effects meta-analysis of the effect of race (Black vs White) on JAK2VF CHIP prevalence yielded a meta-analyzed multivariable-adjusted OR of 0.88 (95% CI, 0.43-1.81; P = .73).
Jak2VF CH increases platelet reactivity and accelerates arterial thrombosis
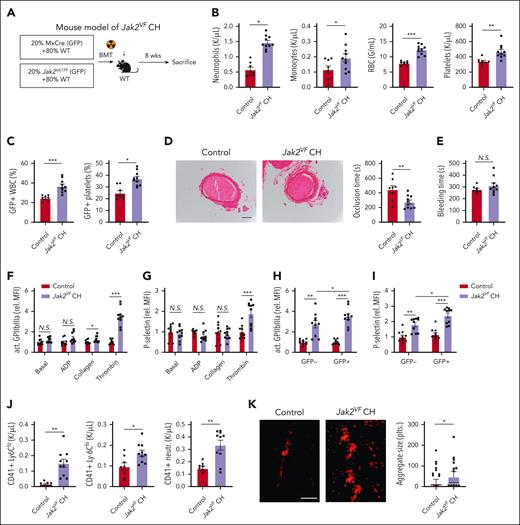
Studies suggest that VAF ≥ 10% in CH (corresponding to 20% of nucleated blood cells as most mutations are heterozygous) significantly increases CVD risk.2,13 To explore the impact of JAK2VF CH on arterial thrombosis, we subjected lethally irradiated WT mice to transplantation with a mixture of bone marrow cells containing 80% WT bone marrow and 20% Mx1-Cre Jak2VF (GFP+) (Jak2VF CH) or 20% Mx1-Cre (GFP+) bone marrow (control; Figure 2A), with induction of Jak2VF expression in donor mice by polyinosinic:polycytidylic acid injection before bone marrow transplantation. Eight weeks after bone marrow reconstitution, Jak2VF CH recipients displayed increased neutrophil, monocyte, red blood cell (RBC), and platelet counts (Figure 2B). Flow cytometry analysis of GFP+CD45+ white blood cells (WBCs) showed a moderate clonal expansion of Jak2VF cells, with ∼38% of Jak2VF cells compared with ∼22% in control recipients (Figure 2C). A similar expansion of GFP+Jak2VF platelets was observed in Jak2VF CH vs control mice (Figure 2C).
Accelerated arterial thrombosis and increased platelet activity in Jak2VF CH. (A) Experimental design of Jak2VF CH mouse model. (B) Peripheral blood cell counts. (C) Jak2VF chimerism in peripheral blood as indicated by fraction of GFP+ WBCs and platelets. (D) FeCl3-induced carotid artery thrombotic occlusion time (seconds). Representative hematoxylin and eosin staining of thrombi were showed. Scale bar, 100 μm. (E) Tail bleeding time (seconds). (F-G) Platelet activation as measured by levels of active GPIIbIIIa conformation (JON/A, F) and P-selectin surface expression (G) of whole blood platelets at baseline or stimulated with platelet agonists adenosine diphosphate (ADP; 10 μM), collagen (0.5 μM) or thrombin (0.5 U/mL). (H-I) Differential analysis of activation in GFP+ (mutated in Jak2VF CH) and GFP⁻ thrombin-stimulated platelets (GPIIbIIIa activation/JON/A, H; P-selectin) (I). (J) Platelet-leukocyte heteroaggregates in the peripheral blood, indicated by number of CD41+ Ly6C-low and Ly6C-high monocytes, and neutrophils. (K) Representative immunofluorescence-stained fluorescein isothiocyanate–labeled anti-CD41 images. Platelet adhesion on collagen surface under arterial flow ex vivo (scale bar, 50 μm; aggregate size was quantified from 100 aggregates from 4 different animals and presented as median and 10 to 90 percentile). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; Student t test or Mann-Whitney U test, as appropriate; N.S., not significant.
Accelerated arterial thrombosis and increased platelet activity in Jak2VF CH. (A) Experimental design of Jak2VF CH mouse model. (B) Peripheral blood cell counts. (C) Jak2VF chimerism in peripheral blood as indicated by fraction of GFP+ WBCs and platelets. (D) FeCl3-induced carotid artery thrombotic occlusion time (seconds). Representative hematoxylin and eosin staining of thrombi were showed. Scale bar, 100 μm. (E) Tail bleeding time (seconds). (F-G) Platelet activation as measured by levels of active GPIIbIIIa conformation (JON/A, F) and P-selectin surface expression (G) of whole blood platelets at baseline or stimulated with platelet agonists adenosine diphosphate (ADP; 10 μM), collagen (0.5 μM) or thrombin (0.5 U/mL). (H-I) Differential analysis of activation in GFP+ (mutated in Jak2VF CH) and GFP⁻ thrombin-stimulated platelets (GPIIbIIIa activation/JON/A, H; P-selectin) (I). (J) Platelet-leukocyte heteroaggregates in the peripheral blood, indicated by number of CD41+ Ly6C-low and Ly6C-high monocytes, and neutrophils. (K) Representative immunofluorescence-stained fluorescein isothiocyanate–labeled anti-CD41 images. Platelet adhesion on collagen surface under arterial flow ex vivo (scale bar, 50 μm; aggregate size was quantified from 100 aggregates from 4 different animals and presented as median and 10 to 90 percentile). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; Student t test or Mann-Whitney U test, as appropriate; N.S., not significant.
We next assessed the impact of Jak2VF CH on ferric chloride (FeCl3)–induced carotid artery thrombosis. The time to complete occlusion of carotid artery was markedly decreased in Jak2VF CH compared with that in control mice (Figure 2D), indicating accelerated arterial thrombosis. The thrombi were stable because we did not detect signs of instability, such as intermittent recovery of blood flow after initial occlusion or attenuated thrombosis as reported in some studies of panhematopoietic Jak2VF expression.14-16 When tested in male mice with 20% Jak2VF CH, carotid artery thrombosis was similarly accelerated (supplemental Figure 3A). Unlike some reports that showed increased bleeding time in panhematopoietic Jak2VF mouse models,15,16 tail vein bleeding time was comparable between Jak2VF CH and control mice (Figure 2E).
To address the mechanisms of accelerated thrombosis, we evaluated changes of platelet reactivity in Jak2VF CH mice. We treated platelets in whole blood with adenosine diphosphate, collagen, or thrombin. In the resting condition, flow cytometry analysis showed comparable P-selectin surface exposure (surrogate for α granule release) and active integrin αIIbβ3 levels (JON/A binding) in control and Jak2VF CH platelets (Figure 2F-G). The platelets from Jak2VF CH mice exhibited enhanced integrin activation after collagen stimulation and P-selectin exposure as well as increased integrin activation after thrombin stimulation, in comparison with the platelets from control mice (Figure 2F-G). Although the highest activation was found in Jak2VF platelets from Jak2VF CH mice, thrombin-induced activation was also enhanced in WT platelets from Jak2VF CH mice as compared with WT platelets from the control mice (Figure 2H-I; supplemental Figure 3B). Jak2VF platelets also formed more aggregates with myeloid leukocytes in mouse peripheral blood (Figure 2J). To further evaluate changes of platelet function, we assessed adhesion and aggregation of platelets in a collagen-coated flow chamber in a setting simulating arteriolar shear, in which platelet aggregation was significantly increased in Jak2VF CH (Figure 2K). This showed that platelets from Jak2VF CH mice display enhanced activation; this includes both WT and Jak2VF platelets, implying mutant-to-WT platelet cross talk that increases WT platelet activation in Jak2VF CH mice.
Platelet activation is a hallmark of hyperlipidemia and drives atherosclerotic arterial disease.17 To assess Jak2VF CH–dependent platelet activation in hyperlipidemic mice, we subjected lethally irradiated Ldlr-deficient (Ldlr-/-) recipient mice to transplantation with bone marrow cells containing 80% WT bone marrow and 20% Mx1-Cre/Jak2VF (Jak2VF CH) or 20% Mx1-Cre bone marrow (control). Eight weeks after transplantation, mice were subjected to a high-fat, high-cholesterol western diet (WD) for 16 weeks (supplemental Figure 3C). Peripheral blood cell counts showed increased leukocyte and platelet counts and hematocrit in Jak2VF CH mice as compared with in controls (supplemental Figure 3D-E). Platelet surface glycoprotein (GP) IIb and VI showed comparable expression between 2 groups (supplemental Figure 3F). Similar to Jak2VF CH mice on a chow diet, hyperlipidemic Jak2VF mice displayed increased integrin αIIbβ3 activation and higher P-selectin surface expression after thrombin stimulation (supplemental Figure 3G). Platelets of mice with Jak2VF also formed more aggregates with neutrophils in peripheral blood compared with those of control mice (supplemental Figure 3H). Thus, Jak2VF drives platelet activation in both normolipidemic and hyperlipidemic mice.
Recent studies suggest that individuals with low JAK2VF burden (VAF < 2%) could have an increased risk of CVD18 or ischemic stroke.19 Therefore, we carried out a study to explore the role of low allele burden Jak2VF (LAB Jak2VF) on atherothrombosis. We reconstituted Ldlr−/− bone marrow recipients with a mixture of 1.5% GFP+Jak2VF and 98.5% WT bone marrow (LAB Jak2VF) or 1.5% Mx1-Cre/GFP+ and 98.5% WT bone marrow (control; supplemental Figure 4A). After 16 weeks of WD, the 2 groups of mice showed similar blood cell profiles with no significant differences in RBC, platelet, neutrophil, or monocyte counts (supplemental Figure 4B). GFP+ leukocyte and platelet counts were similar in LAB Jak2VF and control groups, with GFP+ leukocytes and platelets of 2% to 3% after 16 weeks of WD (supplemental Figure 4C). The spleen weight was also similar in these 2 groups (supplemental Figure 4D). Without any overt MPN phenotypes, LAB Jak2VF mice still showed accelerated carotid thrombosis (supplemental Figure 4E) as well as increased platelet reactivity to thrombin (supplemental Figure 4F-G). Interestingly, both Jak2VF and WT platelets from LAB Jak2VF mice showed increased activation in response to thrombin when compared with their counterpart platelets from the control mice (supplemental Figure 4H-I), providing additional support for the prothrombotic cross talk between mutant and WT platelets. These findings also indicate the prothrombotic effect of Jak2VF CH, even at LAB without changes of blood cell counts.
There is evidence that neutrophils in JAK2VF-mediated MPNs are primed for neutrophil extracellular trap (NET) formation, which promotes venous thrombosis in mouse models of MPNs.6 To explore the potential role of NETosis in thrombotic Jak2VF CH, arterial thrombi were stained with neutrophil marker Ly6G and citrullinated histone H3. However, comparable NET signals and neutrophil abundance were found in the thrombi from Jak2VF CH and control mice (supplemental Figure 5A).
Jak2VF CH promotes the production of young hyperreactive platelets
Reticulated platelets are newly released from megakaryocytes (MKs) with larger size and higher RNA content, which in comparison with mature platelets are more reactive and exhibit higher thrombogenicity. Elevated reticulated platelets are a biomarker for adverse cardiovascular events and mortality in patients with CVD.20JAK2VF has been linked to the increased fraction and quantity of reticulated platelets in patients with MPN, which might contribute to the prothrombotic phenotype in these patients. Further myelosuppressive treatment in MPN showed improved outcomes dependent on reduction of young platelets.21 It is not clear whether the fraction and/or quantity of young platelets are altered in JAK2V617F CH and whether there is altered platelet production. We evaluated platelet parameters in association with JAK2VF CH. Analysis of the UK Biobank cohort showed that JAK2VF CH was associated with increased MPV as well as platelet crit and platelet distribution width, after adjustment for platelet counts (Figure 3A; supplemental Figure 6A-B). These data suggest increase in platelet production and number of young platelets in JAK2VF CH.
Accelerated production of hyperreactive young platelets in Jak2VF CH. (A) Effect of JAK2VF on platelet indices in the UK Biobank cohort adjusted to platelet counts (in addition to age, sex, square of age, smoking status, first 5 genetic principal components, graph shows effect size as SD with 95% CI). (B) Fraction of young (reticulated) platelets. (C) Platelet production rate per hour. (D) Relative platelet life span. (E) Platelet size. (F-L) Intravital bone marrow imaging of thrombopoiesis in Jak2VF CH and control mice. (F) Experimental design and representative Alexa 488 anti-mouse glycoprotein IX– and phycoerythrin anti-mouse Ly-6G–labeled images (arrow indicates proplatelets [PPs], and arrowhead indicates MKs, green color; red color shows neutrophils; see also supplemental Videos). Analysis of PP release relative to MK numbers (G), MK size (H), relative MK number (I). (J-L) Frequency distribution or abundancy of different PP-releasing MK-subgroups (3pp-MK most PP producing, 1pp-MK least PP producing) (J) and their contribution to MK size (K) and PP-release time (L). (M-N) Relative activation of young as compared with old platelets shown for thrombin-induced P-Selectin surface expression (M), GPIIbIIIa activation (JON/A, N) and binding of fibrinogen (O) in Jak2VF CH and control mice. (P) Contribution of young platelets to platelet-leukocyte heteroaggregates in the whole blood of Jak2VF CH mice. In panels H, K, and L, data points present single MKs in different bone marrow areas from 4 different animals. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; Student t test or Mann-Whitney U test, as appropriate. PMA, platelet-monocyte aggregation; PNA, platelet-neutrophil aggregation.
Accelerated production of hyperreactive young platelets in Jak2VF CH. (A) Effect of JAK2VF on platelet indices in the UK Biobank cohort adjusted to platelet counts (in addition to age, sex, square of age, smoking status, first 5 genetic principal components, graph shows effect size as SD with 95% CI). (B) Fraction of young (reticulated) platelets. (C) Platelet production rate per hour. (D) Relative platelet life span. (E) Platelet size. (F-L) Intravital bone marrow imaging of thrombopoiesis in Jak2VF CH and control mice. (F) Experimental design and representative Alexa 488 anti-mouse glycoprotein IX– and phycoerythrin anti-mouse Ly-6G–labeled images (arrow indicates proplatelets [PPs], and arrowhead indicates MKs, green color; red color shows neutrophils; see also supplemental Videos). Analysis of PP release relative to MK numbers (G), MK size (H), relative MK number (I). (J-L) Frequency distribution or abundancy of different PP-releasing MK-subgroups (3pp-MK most PP producing, 1pp-MK least PP producing) (J) and their contribution to MK size (K) and PP-release time (L). (M-N) Relative activation of young as compared with old platelets shown for thrombin-induced P-Selectin surface expression (M), GPIIbIIIa activation (JON/A, N) and binding of fibrinogen (O) in Jak2VF CH and control mice. (P) Contribution of young platelets to platelet-leukocyte heteroaggregates in the whole blood of Jak2VF CH mice. In panels H, K, and L, data points present single MKs in different bone marrow areas from 4 different animals. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; Student t test or Mann-Whitney U test, as appropriate. PMA, platelet-monocyte aggregation; PNA, platelet-neutrophil aggregation.
We assessed blood platelet–related kinetics in Jak2VF CH mice. Platelet production rate was markedly increased, resulting in increased young platelet fraction and quantity (Figure 3B-C), whereas platelet life span was decreased in Jak2VF CH (Figure 3D) indicating accelerated platelet turnover. Besides, platelet size was increased in Jak2VF CH (Figure 3E). Proplatelet formation and release from MKs accounts for platelet production and was assessed by 2-photon excitation fluorescence microscopy in vivo (Figure 3F; supplemental Videos). MK proplatelet formation and MK size were increased in Jak2VF CH mice (Figure 3G-H). Although MK were relatively decreased among bone marrow cells (Figure 3I), the abundance of proplatelet-forming MK with high release rate was higher and proplatelet release per minute was increased in Jak2VF CH mice (Figure 3J-L).
To assess whether young platelets were more active, we measured surface levels of P-selectin and adhesion receptors of young reticulated or mature platelets from Jak2VF CH mice. The increases in thrombin-induced surface P-selectin and GPIIbIIIa activation in Jak2VF CH were more pronounced in young platelets than in mature platelets (Figure 3M-N). Young platelets in Jak2VF CH further showed slightly increased basal GPIb and GPVI expression (supplemental Figure 6C-D). Consistently, increased fibrinogen binding in Jak2VF CH was triggered by young, reticulated platelets (Figure 3O) that disproportionately contributed to platelet-leukocyte aggregate formation (Figure 3P). Together, our data indicate increased generation and higher reactivity of young platelets in Jak2VF CH mice, which likely contribute to the overall increased platelet activation.
Selective Jak2VF expression in MK and platelets promotes arterial thrombosis
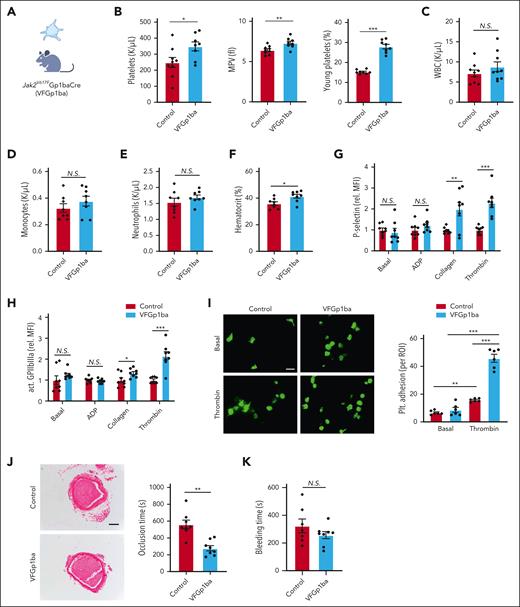
To determine whether Jak2VF expression in the MK or platelet lineage contributes to platelet activation and accelerated arterial thrombosis, we attempted to restrict Jak2VF expression to MK and platelets using the Gp1ba-Cre system (VFGP1ba; Figure 4A).12 Compared with littermate Gp1ba-Cre controls, VFGp1ba mice displayed an increase in platelet counts, MPV, and an increased fraction of young platelets (Figure 4B). In contrast to Jak2VF CH, total WBC, neutrophil, and monocyte counts showed no changes (Figure 4C-E). However, hematocrit was also moderately increased in VFGP1ba mice (Figure 4F), consistent with a minor leaky expression in erythroid lineage as reported with Gp1ba-Cre–directed floxed-gene expression.12 However, this increase in hematocrit was much less than that in 20% Jak2VF CH. Similar to Jak2VF CH mice, VFGp1ba increased platelet surface P-selectin and active αIIbβ3 levels after collagen and thrombin stimulation (Figure 4G-H). Consistently, after thrombin stimulation VFGp1ba platelet adhesion was increased relative to the control platelets (Figure 4I). Importantly, time to complete carotid artery occlusion was shortened by ∼50% in VFGp1ba mice (Figure 4J), comparable with that in Jak2VF CH mice, whereas tail vein bleeding was not altered (Figure 4K). Besides, NETosis in thrombotic lesions showed no change in VFGp1ba mice relative to the control (supplemental Figure 5B). These findings indicate that Jak2VF expression in platelets promotes platelet activation and arterial thrombosis and suggests a major role of increased platelet activation in accelerated arterial thrombosis in Jak2VF CH mice.
Accelerated arterial thrombosis and increased platelet activity in VF-Gp1ba mice. (A) MK lineage–specific expression of Jak2VF mouse model. (B) Platelet count (left), MPV (middle) and young platelet fraction (right). (C-F) Differential peripheral WBC count and hematocrit. (G-H) Platelet activation as measured by P-selectin surface expression (G) and GPIIbIIIa activation (JON/A, H) level of whole blood platelets at baseline or stimulated with platelet agonists ADP (10 μM), collagen (0.5 μM) or thrombin (0.5 U/mL). (I) Representative immunofluorescence-stained fluorescein isothiocyanate–labeled phalloidin images. Adhesion of platelets to fibrinogen-coated chamber slides with or without thrombin stimulation (0.5 U/mL). Scale bar, 5 μm. (J) FeCl3-induced carotid artery thrombotic occlusion time (seconds). Representative hematoxylin and eosin staining of thrombi were showed. Scale bar, 100 μm. (K) Tail bleeding time (seconds). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; Student t test or Mann-Whitney U test as appropriate.
Accelerated arterial thrombosis and increased platelet activity in VF-Gp1ba mice. (A) MK lineage–specific expression of Jak2VF mouse model. (B) Platelet count (left), MPV (middle) and young platelet fraction (right). (C-F) Differential peripheral WBC count and hematocrit. (G-H) Platelet activation as measured by P-selectin surface expression (G) and GPIIbIIIa activation (JON/A, H) level of whole blood platelets at baseline or stimulated with platelet agonists ADP (10 μM), collagen (0.5 μM) or thrombin (0.5 U/mL). (I) Representative immunofluorescence-stained fluorescein isothiocyanate–labeled phalloidin images. Adhesion of platelets to fibrinogen-coated chamber slides with or without thrombin stimulation (0.5 U/mL). Scale bar, 5 μm. (J) FeCl3-induced carotid artery thrombotic occlusion time (seconds). Representative hematoxylin and eosin staining of thrombi were showed. Scale bar, 100 μm. (K) Tail bleeding time (seconds). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; Student t test or Mann-Whitney U test as appropriate.
Erythroid lineage expression of Jak2VF promotes arterial thrombosis
Histological analysis of FeCl3-induced thrombi in Jak2VF CH mice showed increased density of RBCs as compared with controls (supplemental Figure 7A). To assess the contribution of erythroid Jak2VF expression to thrombosis, we used EpoR-Cre to restrict Jak2VF expression in erythroid lineage (VFEpoR) and found that Jak2VF RBCs formed more aggregates with platelets in peripheral blood compared with those in control mice (supplemental Figure 7B). As we have previously reported, red-cell distribution width, a known marker of CVD risk,8,22 was increased in VFEpoR mice.8 Relative to controls, VFEpoR mice showed a small but significant acceleration of arterial thrombosis (supplemental Figure 7C). To assess the impact of increased hematocrit, a selective increase in RBC biogenesis and hematocrit in VFEpoR mice without impact on WBC and platelet counts was induced using low-dose erythropoietin (EPO), as reported.8 Low-dose EPO further accelerated arterial thrombosis in VFEpoR mice but had no significant effect in control mice (supplemental Figure 7C). The acceleration of thrombosis in EPO-treated VFEpoR mice (29%) appeared to be less than that in 20% Jak2VF CH mice (38%) or VFGp1ba mice (50%), although hematocrit levels went in the opposite direction (VFEpoR > 20% Jak2VF CH > VFGp1ba; supplemental Figure 7D), consistent with a major prothrombotic role of platelets in Jak2VF CH. As in Jak2VF CH mice, thrombi from VFEpoR mice also displayed an increased content of RBC islands, particularly in the peripheral region, and this increase was more pronounced after EPO induced erythrocytosis (supplemental Figure 7E). These findings indicate that erythroid Jak2VF expression also contributes to arterial thrombosis.
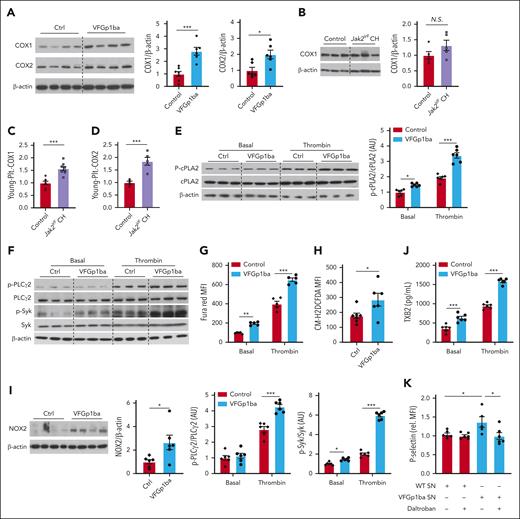
Increased COX-1 and COX-2 expression and cPLA2 activation in Jak2VF platelets
We next performed studies to explore the intrinsic mechanism of increased platelet reactivity in Jak2VF platelets and in particular to determine how this might lead to increased activation of WT platelets in Jak2VF CH. Aspirin has been successfully used as the primary prevention of atherothrombotic events in patients with MPN,23 indicating the critical role of COX-1 and TXA2 pathway. In addition, aspirin resistance is common in MPNs and increased expression of COX-2 in combination with faster renewal of unacetylated COX-1 in newly synthesized platelets appears to contribute to aspirin resistance in patients with essential thrombocythemia (ET).24 Therefore, we assessed platelet COX-1 and COX-2 expression. There was a 2.8-fold increased COX-1 expression in VFGp1ba platelets (Figure 5A), consistent with the evidence of increased expression of PTGS1, the gene encoding COX-1 and among the top upregulated genes in blood cells from patients with MPN.25 VFGp1ba platelets also showed an approximately twofold increase in COX-2 expression (Figure 5A). We next tested and validated these findings using Jak2VF CH mice. We found no significant difference in COX-1 levels in total platelets from Jak2VF CH mice despite an increasing trend (Figure 5B). However, COX-1 and COX-2 expression were significantly increased in young platelets in Jak2VF CH mice (Figure 5C-D). These findings suggest that the enhanced expression and activity of COX-1 and COX-2 may contribute to increased platelet reactivity in Jak2VF platelets and consequent thromboxane-mediated cross talk to WT platelets.
Increased cyclooxygenase expression and thromboxane A2 secretion in Jak2VF platelets. (A) Immunoblot and quantification of COX-1 and COX-2 expression in resting platelets lysates of VF-Gp1ba mice and control mice. (B) Immunoblot and quantification of COX1 expression in platelets lysates from mice with Jak2VF CH or respective controls. (C-D) COX 1 (C) and COX2 expression (D) in young platelets of Jak2VF CH or control mice (assessed by flow cytometry). (E) Immunoblot and quantification of p-cPLA2 expression in resting and thrombin-stimulated (0.5 U/mL; 5 minutes) platelets lysates of VF-Gp1ba mice and control mice. (F) Immunoblot and quantification of p-PLCγ2 and p-Syk expression in resting and thrombin-stimulated (0.5 U/mL; 5 minutes) platelets lysates of VF-Gp1ba mice and control mice. (G) Ca2+ in resting and thrombin-stimulated platelets (0.5 U/mL; 5 minutes) was assessed by Fura Red staining and analyzed by flow cytometry as mean fluorescence intensity (MFI). (H) ROS in platelets was assessed by H2DCFDA staining and analyzed by flow cytometry as MFI. (I) Immunoblot and quantification of NOX2 expression in platelets lysates, which was normalized to β-actin. (J) TXB2 (stable metabolic product of TXA2) detected in supernatant of isolated platelets at baseline or after stimulation with 0.5 U/mL. (K) P-selectin surface expression on WT platelets treated with supernatant (SN) from stimulated Ctrl and Jak2VF platelets with or without preincubation with TXA2 receptor antagonist Daltroban (1 μM). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; Student t test or Mann-Whitney U test or 2-way analysis of variance as appropriate.
Increased cyclooxygenase expression and thromboxane A2 secretion in Jak2VF platelets. (A) Immunoblot and quantification of COX-1 and COX-2 expression in resting platelets lysates of VF-Gp1ba mice and control mice. (B) Immunoblot and quantification of COX1 expression in platelets lysates from mice with Jak2VF CH or respective controls. (C-D) COX 1 (C) and COX2 expression (D) in young platelets of Jak2VF CH or control mice (assessed by flow cytometry). (E) Immunoblot and quantification of p-cPLA2 expression in resting and thrombin-stimulated (0.5 U/mL; 5 minutes) platelets lysates of VF-Gp1ba mice and control mice. (F) Immunoblot and quantification of p-PLCγ2 and p-Syk expression in resting and thrombin-stimulated (0.5 U/mL; 5 minutes) platelets lysates of VF-Gp1ba mice and control mice. (G) Ca2+ in resting and thrombin-stimulated platelets (0.5 U/mL; 5 minutes) was assessed by Fura Red staining and analyzed by flow cytometry as mean fluorescence intensity (MFI). (H) ROS in platelets was assessed by H2DCFDA staining and analyzed by flow cytometry as MFI. (I) Immunoblot and quantification of NOX2 expression in platelets lysates, which was normalized to β-actin. (J) TXB2 (stable metabolic product of TXA2) detected in supernatant of isolated platelets at baseline or after stimulation with 0.5 U/mL. (K) P-selectin surface expression on WT platelets treated with supernatant (SN) from stimulated Ctrl and Jak2VF platelets with or without preincubation with TXA2 receptor antagonist Daltroban (1 μM). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; Student t test or Mann-Whitney U test or 2-way analysis of variance as appropriate.
TXA2 is not stored in platelets; it is synthesized in response to platelet activation by a wide variety of platelet agonists.26 Increased generation of arachidonic acid as the substrate for COX1-mediated TXA2 synthesis is a major underlying mechanism.26 We found that phosphorylation of cytosolic phospholipase A2 (cPLA2), the key enzyme liberating arachidonic acid from membrane phospholipids in platelet activation, was increased 1.4-fold in resting VFGp1ba platelets and 1.8-fold after thrombin stimulation (Figure 5E). cPLA2 activation can occur downstream of phospholipase C activation, inositol 1,4,5-trisphosphate production and calcium mobilization pathway.27,28 Consistently, VFGp1ba platelets showed higher levels of phosphorylated phospholipase Cγ2 after thrombin stimulation (Figure 5F) and increased cytosolic calcium in the resting or stimulated state (Figure 5G). Prior studies indicate that platelet reactive oxygen species (ROS) increase activation of this pathway by oxidative inactivation of SH2 domain–containing PTP-2,29-32 leading to enhanced tyrosine phosphorylation and activation of spleen tyrosine kinase (Syk).30,32 Furthermore, Syk phosphorylations occur and activate PLCγ2.30,33 In parallel, phosphorylation and activation of Syk were increased in VFGp1ba relative to WT platelets in the basal state or in response to thrombin (Figure 5F). NOX2 is critical in ROS-dependent Syk activation during collagen- or thrombin-mediated platelet activation.34 Consistently, ROS were increased in VFGp1ba platelets (Figure 5H) in association with increased NOX2 expression (Figure 5I).
In accordance with higher platelet COX expression in combination with increased cPLA2 activation, production of TXA2 was elevated in resting VFGp1ba platelets, as measured by thromboxane B2, the stable metabolite of TXA2,35 and this increase was further enhanced after stimulation (Figure 5J). To assess the mechanism responsible for a possible prothrombotic cross talk between mutant and WT platelets, WT platelets were stimulated with conditioned media from thrombin-activated VFGp1ba platelets, which led to increased activation as compared with media from thrombin-activated WT platelets. This effect was blocked by daltroban, a thromboxane receptor antagonist (Figure 5K), indicating thromboxane-dependent cross talk between mutant and WT platelets. These findings suggest that increased production of TXA2 by Jak2VF platelets leads to increased activation of WT platelets in Jak2VF CH. Although COX-1 or COX-2 upregulation and increased TXA2 production may not directly mediate platelet responses to thrombin and collagen, TXA2-mediated signaling acts as an amplifying signal for many other platelet agonists, including thrombin and collagen,26 potentially explaining increased platelet activation in response to thrombin and collagen.
Low-dose aspirin reduces arterial thrombosis in VFGp1ba and Jak2VF CH mice
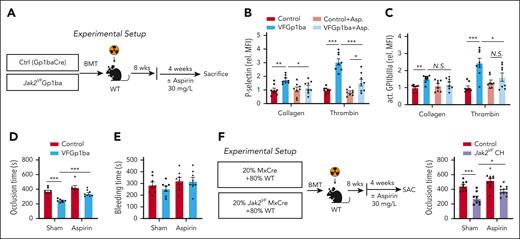
To investigate the functional consequence of elevated platelet COX-1 and COX-2 expression and TXA2 production, we analyzed the effect of low-dose aspirin on platelet reactivity and arterial thrombosis (Figure 6A). The dose used was comparable with that used in low-dose aspirin therapy and clinical trials in humans.36 Four weeks of aspirin treatment did not alter blood cell profiles in control or VFGP1ba mice (supplemental Figure 8A-D), but abrogated hyperreactivity of VFGp1ba platelets in response to collagen or thrombin stimulation (Figure 6B-C). Furthermore, aspirin delayed FeCl3-induced thrombosis in the carotid artery in VFGp1ba mice but had no effect in control mice (Figure 6D). Tail vein bleeding was not influenced (Figure 6E).
Low-dose aspirin treatment reversed accelerated arterial thrombosis and increased platelet activity in Jak2VF CH. (A) Experimental design of aspirin treatment (in the drinking water at 30 mg/L) in MK lineage–specific Jak2VF. (B) Platelet activation as measured by P-selectin surface expression (B) and GPIIbIIIa activation (JON/A, C) of collagen- or thrombin-stimulated platelets of VF-Gp1ba mice after treatment with low-dose aspirin. (D) FeCl3-induced carotid artery thrombotic occlusion time (seconds) and (E) bleeding time in VF-Gp1ba mice or controls. (F) Experimental setup and quantification of FeCl3-induced carotid artery thrombotic occlusion time (seconds) after treatment with low-dose aspirin (in the drinking water at 30 mg/L) in Jak2VF CH and controls. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; Student t test or Mann-Whitney U test or 1-way analysis of variance as appropriate.
Low-dose aspirin treatment reversed accelerated arterial thrombosis and increased platelet activity in Jak2VF CH. (A) Experimental design of aspirin treatment (in the drinking water at 30 mg/L) in MK lineage–specific Jak2VF. (B) Platelet activation as measured by P-selectin surface expression (B) and GPIIbIIIa activation (JON/A, C) of collagen- or thrombin-stimulated platelets of VF-Gp1ba mice after treatment with low-dose aspirin. (D) FeCl3-induced carotid artery thrombotic occlusion time (seconds) and (E) bleeding time in VF-Gp1ba mice or controls. (F) Experimental setup and quantification of FeCl3-induced carotid artery thrombotic occlusion time (seconds) after treatment with low-dose aspirin (in the drinking water at 30 mg/L) in Jak2VF CH and controls. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; Student t test or Mann-Whitney U test or 1-way analysis of variance as appropriate.
We next assessed the impact of low-dose aspirin on thrombosis in Jak2VF CH mice (Figure 6F). Four-week low-dose aspirin treatment did not alter blood cell counts (supplemental Figure 8E-H). Although FeCl3-induced carotid artery thrombosis was accelerated in Jak2VF CH mice and low-dose aspirin significantly reduced arterial thrombosis in Jak2VF CH, it was not same in control mice (Figure 6F). These findings indicate that low-dose aspirin reduces arterial thrombosis in VFGP1ba and Jak2VF CH.
Discussion
We show an increased risk of CVD in association with JAK2VF CHIP likely reflecting atherothrombotic events in a meta-analysis of 3 large cohort studies, including UK Biobank, All of Us, and MGB Biobank, supporting the findings in previous smaller studies.2 Our analyses show that JAK2VF CHIP increases CVD risk (2.4-fold) more than other CHIP mutations (1.09-fold), as suggested in earlier studies.2 The overall CVD risk associated with other CHIP variants was only moderately increased, as suggested by other studies in UK Biobank,4,5 perhaps reflecting the relatively healthy status of the populations studied. The majority of subjects with JAK2VF in these population studies likely do not have overt MPN. However, they may have altered blood cell counts and, thus, may represent an intermediate state between CHIP and MPN. Nonetheless, a further stratification of UK Biobank data by blood cell counts greater or less than 1 SD from mean levels, showed significantly increased CVD risk in both groups, identifying JAK2VF as a bone fide CHIP mutation.
Our studies in mice show that Jak2VF CH promotes accelerated arterial thrombosis both at 20% VAF and 1.5% VAF, and, notably, the latter group did not display significantly altered blood cell counts or splenomegaly and thus may be considered as an authentic non-MPN model. Mechanistically, Jak2VF CH promotes activation of both mutant and WT platelets, driven by the generation of young and more active Jak2VF platelets with increased COX-1 and COX-2 expression and cPLA2 activation. Increased TXA2 production from Jak2VF platelets and thromboxane cross talk from mutant to WT platelets leads to prothrombotic WT platelet priming and activation. Low-dose aspirin reversed increased platelet activation and reduced arterial thrombosis in Jak2VF CH, confirming the causality of COX-1 or COX-2 in accelerated arterial thrombosis and suggesting mechanism-based therapeutic use of low-dose aspirin in the prevention of atherothrombosis in JAK2VF CH.
Our study is the first, to our knowledge, to show accelerated arterial thrombosis in Jak2VF CH mice and suggests a central role of increased Jak2VF platelet activation and cross talk to WT platelets. Prior studies have shown diverse results, with either increased or decreased arterial thrombosis, as well as increased venous thromboembolic disease in panhematopoietic Jak2VF mouse models of MPN.6,14-16,37 An important role of neutrophil NETosis in increased venous thrombosis in Jak2VF MPN mice has been demonstrated,6 and earlier studies have suggested an important role of NETosis in accelerated arterial thrombosis.38 We have shown accelerated thrombosis in Gp1ba-Cre Jak2VF mice without increased WBCs or evidence of increased NETs formation in carotid thrombi, suggesting that the combination of increased platelet activation and erythrocytosis, as occurs in this model, is sufficient to induce accelerated arterial thrombosis.
Although little is known about phenotypic changes of platelets in individuals with JAK2VF CH, several studies have evaluated these changes in JAK2VF MPN patients. Young platelets are elevated and more reactive than their mature counterparts in both patients with polycythemia vera and ET and positively correlate with the JAK2VF mutation.21 Consistent with elevated platelet counts and increased adjusted MPV in individuals with JAK2VF CH, we observed increased overall platelet counts in the Jak2VF CH model driven by markedly increased platelet production resulting in a highly elevated number of young platelets with increased MPV. This was consistent with our findings in the bone marrow of Jak2VF CH mice with abundance of larger and more proplatelet releasing megakaryocytes. Importantly, younger platelets were particularly more activated in Jak2VF CH.
Patients with MPN also show increased urinary TXA2 metabolites consistent with a role of increased platelet TXA2 production and activation.39,40 Our finding of increased platelet COX-1 and COX-2 expression and TXA2 production are consistent with these earlier studies and suggest a platelet intrinsic effect of Jak2VF to promote platelet activation in CH. This leads to the novel observation that increased thromboxane production by mutant platelets leads to priming and activation of WT platelets in CH and likely contributes to the elevated thrombotic risk in JAK2VF CH,6 that is even observed in patients with JAK2VF VAF as low as ≤2%.18,19 The impact of this mechanism in Jak2VF CH might be even more important because increased COX expression was particularly pronounced in young platelets that are increased in Jak2VF CH.
Our findings in Jak2VF CH contrast with reports of reduced or unaltered platelet reactivity in Jak2VF panhematopoietic models.8-10,21 The hemostatic deficit in Jak2VF MPN has been attributed to increased proteolytic cleavage of von-Willebrand factor leading to von-Willebrand factor multimer deficiency.14,16 Although the reason for the apparent phenotypic differences in MPN and CH models remains unclear, our findings suggest that hemostatic defects may be less prominent at lower Jak2VF allele burden.
Low-dose aspirin is widely used in the secondary prevention of CVD to reduce the risk of myocardial infarction and ischemic stroke. However, its use in primary prevention of CVD in the healthy older adults has been called into question by recent studies.41 The Aspirin in Reducing Events in the Elderly trial showed that low-dose aspirin does not improve all-cause mortality or CVD risk but increases the risk of major hemorrhage,36 suggesting the need for more precise therapeutic targeting. Aspirin is considered as the primary choice for prevention of thrombosis in patient with JAK2VF-positive MPNs.23 However, aspirin resistance is common in these patients, probably because of increased young platelet COX-2 expression and faster renewal of unacetylated platelet COX-1 as a result of increased platelet production.24 Indeed, twice-daily or thrice-daily regimens have been shown to improve platelet responses to aspirin and lower serum TXB2 levels.42,43 Our studies showing benefit of low-dose aspirin in thrombosis in Jak2VF CH but not control mice suggest that individuals with JAK2VF CH could also benefit from low-dose aspirin in the prevention of CVD.
Acknowledgments
This work was supported by grants from the National Institutes of Health, National Heart, Lung and Blood Institute (HL148050 [P.N.], HL148071 [N.W.], and HL155431 [A.R.T.]), the Leducq Foundation (TNE-18CVD04) (P.N., S.M., and A.R.T.), the German Centre for Cardiovascular Research (DZHK) and the German Ministry of Education and Research (BMBF) (grants 81Z0600204 [C.S.], MHA 1.4VD [S.M.], and a Postdoc Start-up Grant [J.P.]), the Deutsche Forschungsgemeinschaft (SCHU 2297/1-1 [C.S.], PE2704/3-1 [T.P.], CRC 1123 projects A07 [C.S.] and B06 [S.M.], and CRC TRR332 project A6 [C.S.]), and the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant agreement No. 833440) (S.M.).
Authorship
Contribution: W.L. and J.P. designed and performed experiments, analyzed data, and wrote the manuscript; A.S., M.C.H., T.N., and A.P. performed epidemiological analyses; Q.U.A., M.Y., L.W., T.X., and M.A.H. performed the experiments; Z.Z. and T.P. contributed the in intravital thrombopoiesis model; D.S. generated and provided key antibodies for this intravital microscopy model; P.N., S.M., and A.R.T. shared the reagents, designed experiment, and provided scientific feedback about the manuscript; and C.S. and N.W. designed experiments, wrote the manuscript, supervised, and directed the project.
Conflict-of-interest disclosure: A.R.T. is a consultant for Amgen, CSL Behring, AstraZeneca, and Foresite Laboratories and is on the Science Advisory Board of Staten Biotech, Fortico Biotech, and Beren Therapeutics. P.N. reports research grants from Allelica, Apple, Amgen, Boston Scientific, Genentech, Roche, and Novartis; personal fees from Allelica, Apple, AstraZeneca, Blackstone Life Sciences, Eli Lilly & Co, Foresite Labs, Genentech/Roche, GV, HeartFlow, Magnet Biomedicine, and Novartis; scientific advisory board membership of Esperion Therapeutics, Preciseli, and TenSixteen Bio; scientific cofounding of TenSixteen Bio; equity in MyOme, Preciseli, and TenSixteen Bio; and spousal employment at Vertex Pharmaceuticals, all unrelated to the present work. The remaining authors declare no competing financial interests.
Correspondence: Christian Schulz, Medizinische Klinik und Poliklinik, Klinikum der Universität München, Ludwig-Maximilians-Universität München, Campus Großhadern, Marchioninistr 15, 81377 Munich, Germany; email: christian.schulz@med.uni-muenchen.de; and Nan Wang, Medicine, Columbia University Irving Medical Center, PS 8-401, 630 W. 168th St, New York, NY 10032; email: nw30@cumc.columbia.edu.
References
Author notes
W.L. and J.P. are joint first authors.
Data are available on request from the corresponding authors, Christian Schulz (christian.schulz@med.uni-muenchen.de) and Nan Wang (nw30@cumc.columbia.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Accelerated production of hyperreactive young platelets in Jak2VF CH. (A) Effect of JAK2VF on platelet indices in the UK Biobank cohort adjusted to platelet counts (in addition to age, sex, square of age, smoking status, first 5 genetic principal components, graph shows effect size as SD with 95% CI). (B) Fraction of young (reticulated) platelets. (C) Platelet production rate per hour. (D) Relative platelet life span. (E) Platelet size. (F-L) Intravital bone marrow imaging of thrombopoiesis in Jak2VF CH and control mice. (F) Experimental design and representative Alexa 488 anti-mouse glycoprotein IX– and phycoerythrin anti-mouse Ly-6G–labeled images (arrow indicates proplatelets [PPs], and arrowhead indicates MKs, green color; red color shows neutrophils; see also supplemental Videos). Analysis of PP release relative to MK numbers (G), MK size (H), relative MK number (I). (J-L) Frequency distribution or abundancy of different PP-releasing MK-subgroups (3pp-MK most PP producing, 1pp-MK least PP producing) (J) and their contribution to MK size (K) and PP-release time (L). (M-N) Relative activation of young as compared with old platelets shown for thrombin-induced P-Selectin surface expression (M), GPIIbIIIa activation (JON/A, N) and binding of fibrinogen (O) in Jak2VF CH and control mice. (P) Contribution of young platelets to platelet-leukocyte heteroaggregates in the whole blood of Jak2VF CH mice. In panels H, K, and L, data points present single MKs in different bone marrow areas from 4 different animals. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; Student t test or Mann-Whitney U test, as appropriate. PMA, platelet-monocyte aggregation; PNA, platelet-neutrophil aggregation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/15/10.1182_blood.2023022260/3/m_blood_bld-2023-022260-gr3.jpeg?Expires=1769083685&Signature=OJHrNkmsOYglQWUnbCG2khTUda-79VLRGGBvMSnVYHIAOECys~wscA-4gc1bFNcv5ymmgs9NwTMsdqWry2XQFeWZNSgjzutEv11ol14ewmVnNw0ANfj3OBZd5Eux8BE94z3tfsS~mk7HiZ5xMX952ddMF7eKsWDM2IodQkVbypiprIdjVWJlkswno5DJHbYxH39ghWs5cFe-b9FRx9jN537jKGYSn1vOYBVdywIp3iAkwiI8OPuRc~vaPidcFYIoJgB1G~rQskNq3QUWlN1WRUGRDVpNDxv~katj89LWgMU~OMrOERkeVJYyNnknWXSo73ePbnUbBtopjj3j5YMbMg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal