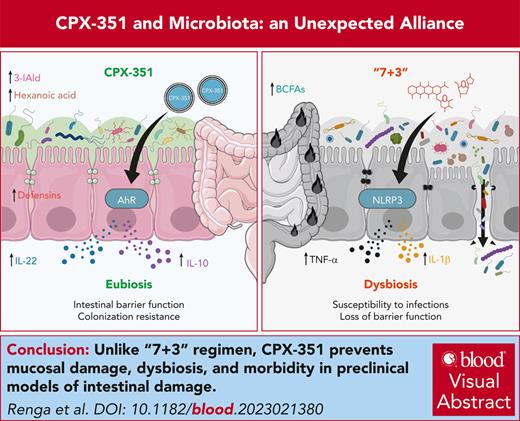
Different from “7 + 3” combination, CPX-351 prevents mucosal damage, dysbiosis, and morbidity in intestinal inflammation.
CPX-351 protects via the aryl hydrocarbon receptor–IL-22-IL-10 host pathway and the production of immunomodulatory metabolites by anaerobes.
Visual Abstract
CPX-351, a liposomal combination of cytarabine plus daunorubicin, has been approved for the treatment of adults with newly diagnosed, therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes, because it improves survival and outcome of patients who received hematopoietic stem cell transplant compared with the continuous infusion of cytarabine plus daunorubicin (referred to as “7 + 3” combination). Because gut dysbiosis occurring in patients with AML during induction chemotherapy heavily affects the subsequent phases of therapy, we have assessed whether the superior activity of CPX-351 vs “7 + 3” combination in the real-life setting implicates an action on and by the intestinal microbiota. To this purpose, we have evaluated the impact of CPX-351 and “7 + 3” combination on mucosal barrier function, gut microbial composition and function, and antifungal colonization resistance in preclinical models of intestinal damage in vitro and in vivo and fecal microbiota transplantation. We found that CPX-351, at variance with “7 + 3” combination, protected from gut dysbiosis, mucosal damage, and gut morbidity while increasing antifungal resistance. Mechanistically, the protective effect of CPX-351 occurred through pathways involving both the host and the intestinal microbiota, namely via the activation of the aryl hydrocarbon receptor–interleukin-22 (IL-22)–IL-10 host pathway and the production of immunomodulatory metabolites by anaerobes. This study reveals how the gut microbiota may contribute to the good safety profile, with a low infection-related mortality, of CPX-351 and highlights how a better understanding of the host-microbiota dialogue may contribute to pave the way for precision medicine in AML.
Introduction
CPX-351, a liposomal combination of cytarabine plus daunorubicin at a fixed 5:1 synergistic molar ratio,1 was approved in 2017 for the treatment of adults with newly diagnosed, therapy-related acute myeloid leukemia (t-AML) or AML with myelodysplasia-related changes for improved overall survival,2-4 compared with induction chemotherapy with a continuous 7-day infusion of cytarabine plus daunorubicin (hereafter referred to as “7 + 3” combination).3 In the face of incidence of nonhematologic adverse events that were either comparable between CPX-351 and “7 + 3” combination2 or reduced (50% reduction of gastrointestinal toxicity5), a decidedly lower mortality rate from infection (3.5% vs 7.3%) was observed with CPX-351.2,3 More recently, we confirmed the good safety profile of CPX-351 in a real-life setting, with low incidence of fungal infections and low infection-related mortality.6
In recent years, the interaction between microbiota and anticancer drugs is drawing growing interest, because the setup of interventions is aimed at shaping microbiota to optimize drug efficacy and reduce side effects.7 Dysbiosis is not only associated with local adverse effects and serious infections but could also influence the efficacy of chemotherapy as well as promote the emergence of antimicrobial and antitumoral drug resistance.8-10 Intriguingly, it has recently been reported that a loss of microbiota-derived protective metabolites would predict the onset of neutropenic fever, a finding suggesting a revision of the current antimicrobial approach to neutropenic fever.11 On the other side, antineoplastic chemotherapy indirectly amplifies dysbiosis and has profound effects mainly on the intestinal epithelium, influencing cancer progression, treatment efficacy, and toxicity. Studies have shown that the gut microbiota of patients with AML is disrupted during induction chemotherapy,12-14 being dysbiosis relevant for progression and subsequent phases of therapy,15 including hematopoietic stem cell transplantation, a clinical setting in which a lower intestinal microbial diversity before transplantation is associated with poor survival.16-18 Thus, a better understanding of the host-microbiota interactions in disease and therapy may likely help chemotherapy optimization in patients with AML.
Based on these premises, we resorted to a translational study to verify whether the better profile of CPX-351 implicates an action on and by the intestinal microbiota. To this purpose, we have assessed the effects of CPX-351 compared with the “7 + 3” combination on mucosal barrier function, gut microbial composition and function, and antifungal colonization resistance in preclinical models of intestinal damage in vitro and in vivo and fecal microbiota transplantation. We found that CPX-351, at variance with “7 + 3” combination, protected from gut dysbiosis, mucosal damage, and gut morbidity while increasing antifungal resistance.
Materials and methods
In vitro studies
Differentiated CaCo-2 cells were exposed to the “7 + 3” combination, CPX-351 or empty liposomes as described19 (see the supplemental Data, available on the Blood website, for details and assays). AhR activity was assessed on the reporter H1L6.1c3 cell line as detailed in the supplemental Data.
In vivo studies
Female mice aged 8 to 10 weeks were treated IV for 3 times every 3 days with the “7 + 3” combination or CPX-351, as suggested1 as well as with empty lyposomes and 3-IAld as described in supplemental Data. Dextran sulfate sodium (DSS) colitis and infections are described in the supplemental Data. Fecal microbiota transplantation was done as described.20 C57BL/6 mice were from Charles River; Il10–/–, B6.129-Ahrtm1Bra/J Ahr-deficient (Ahr–/–) NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were bred under specific pathogen-free conditions in the Animal Facility of Perugia (Italy).
Metagenomics
The bacterial microbiota was amplified on the V4–V5 hypervariable regions of the 16S ribosomal RNA (rRNA) and was sequenced on a 300 bp paired-end read sequencing on the Illumina MiSeq. High quality primer-clipped sequences were imported and analyzed in the next-generation microbiome bioinformatics Qiime2 platform. The Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt2) pipeline was then applied to predict metagenome functions (EC number and KEGG Orthology databases) from 16S samples.
Metabolomics
Metabolites were determined in fecal and blood samples by high-performance liquid chromatography and tandem mass spectrometry as described in supplemental Data.
Statistical analysis
Statistical significance was calculated by a 1-way or 2-way analysis of variance (the Bonferroni post hoc test). Data are pooled results (mean ± standard errors of the mean) or representative images from 2 or 3 experiments. The beta diversities were compared using a permanova test on both all and pairwise samples; significance of their corresponding principal coordinate analyses (PCoAs) was evaluated by the Wilcoxon multiple-comparison test. The in vivo groups consisted of 4 to 6 mice per group. GraphPad Prism software V.6.01 (GraphPad Software) was used for analysis.
Results
CPX-351 protects from intestinal pathology
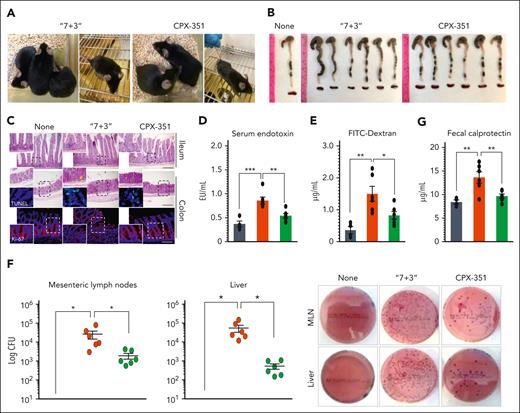
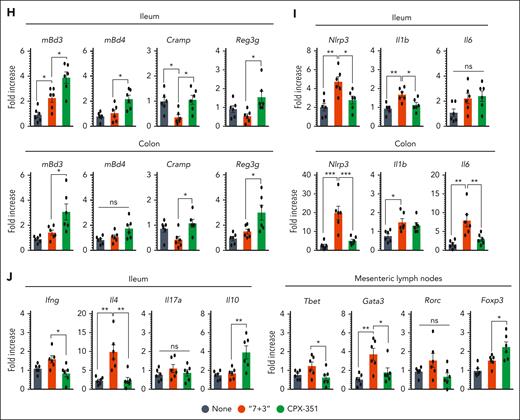
To assess the impact of CPX-351 or “7 + 3” combination on intestinal epithelial barrier function, permeability, and immune and microbial homeostasis, we injected female C57BL/6 mice IV every 3 days for 3 times with 12:5.3 mg/kg CPX-351 or 600:9 mg/kg of “7 + 3” combination, the maximum tolerated dose, as already described in the leukemic model1 and confirmed in our dose-dependent study in naïve mice (supplemental Figure 1). Upon inspection, as opposed to the lack of pathology observed upon CPX-351 treatment, signs of morbidity and intestinal pathology such as stiffness, immobility, (Figure 1A) and reduced colon length (Figure 1B) were observed upon “7 + 3” treatment along with the presence of numerous inflammatory infiltrates in the ileum and colon (Figure 1C), with signs of diffuse cellular damage and reduced intestinal cell proliferation (Figure 1C, insets). An increased epithelial paracellular permeability, as revealed by the elevated serum content of endotoxin (Figure 1D) and the gavaged fluorescein isothiocyanate–dextran (Figure 1E), as well as the translocation of bacteria to the mesenteric lymph nodes and liver (Figure 1F), were also observed. The local immune competence was also impaired by the “7 + 3” treatment but maintained by CPX-351, as revealed by the reduced levels of fecal calprotectin (Figure 1G), increased expression of genes of Bd3 and Bd4 defensins, the cathelicidin-related antimicrobial peptide, and the regenerating islet-derived 3-gamma protein (Figure 1H) in CPX-351–treated mice, thus suggesting an activity of CPX-351 on epithelial colonization resistance. Moreover, at variance with the “7 + 3” treatment, CPX-351 did not promote the expression of the inflammatory receptor NLRP3 and downstream inflammatory interleukin-1β (IL-1β) and IL-6 (Figure 1I), suggesting that the local inflammation is promoted by “7 + 3” combination but not CPX-351. Accordingly, CPX-351, although not affecting the Rorc+Th17/IL-17A pathway, failed to activate the inflammatory Tbet+Th1/IFN-γ and GATA3+Th2/IL-4 pathways, while promoting the FoxP3+regulatory T cells (Treg)/IL-10 anti-inflammatory pathway (Figure 1J). Thus, CPX-351 does not induce intestinal toxicity and immune dysregulation that are instead both observed by the “7 + 3” combination, recently found to have a disruptive effect of on the epithelial barrier function.21
CPX-351 does not promote intestinal pathology. Effects of the “7 + 3” combination or CPX-351 administration every 3 days for 3 times in C57BL/6 mice. Mice were evaluated for (A) clinical signs of morbidity, (B) intestinal length, and (C) histopathology (PAS staining) and epithelial damage (TUNEL assay and Ki-67 staining in the insets), intestinal permeability by (D) serum endotoxin, (E) Fluorescein isothiocyanate (FITC)–dextran fluorescence, and (F) bacterial translocation to mesenteric lymph nodes and liver, (G) fecal calprotectin and expression (by reverse transcription polymerase chain reaction) of genes associated with (H) epithelial barrier function, (I) inflammatory cytokines and NLRP3 and (J) adaptive T helper cell responses in the mesenteric lymph nodes and ileum. Yellow arrows indicate inflammatory foci (magnified in the insets). Data are representative of 2 or 3 independent experiments. Each in vivo experiment includes 4 to 6 mice per group. Data are represented as mean ± standard error of the mean (SEM). For immunofluorescence, nuclei were counterstained with Hoechst. Photographs were taken with a high-resolution microscope (Olympus BX51); original magnification ×20 (C, ileum; scale bar, 200 μm) and ×40 (C, colon; scale bar, 100 μm). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; treated vs untreated (None) mice and CPX-351 vs “7 + 3” treatment. One-way analysis of variance (ANOVA), Bonferroni multiple comparison test. ns, not significant.
CPX-351 does not promote intestinal pathology. Effects of the “7 + 3” combination or CPX-351 administration every 3 days for 3 times in C57BL/6 mice. Mice were evaluated for (A) clinical signs of morbidity, (B) intestinal length, and (C) histopathology (PAS staining) and epithelial damage (TUNEL assay and Ki-67 staining in the insets), intestinal permeability by (D) serum endotoxin, (E) Fluorescein isothiocyanate (FITC)–dextran fluorescence, and (F) bacterial translocation to mesenteric lymph nodes and liver, (G) fecal calprotectin and expression (by reverse transcription polymerase chain reaction) of genes associated with (H) epithelial barrier function, (I) inflammatory cytokines and NLRP3 and (J) adaptive T helper cell responses in the mesenteric lymph nodes and ileum. Yellow arrows indicate inflammatory foci (magnified in the insets). Data are representative of 2 or 3 independent experiments. Each in vivo experiment includes 4 to 6 mice per group. Data are represented as mean ± standard error of the mean (SEM). For immunofluorescence, nuclei were counterstained with Hoechst. Photographs were taken with a high-resolution microscope (Olympus BX51); original magnification ×20 (C, ileum; scale bar, 200 μm) and ×40 (C, colon; scale bar, 100 μm). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; treated vs untreated (None) mice and CPX-351 vs “7 + 3” treatment. One-way analysis of variance (ANOVA), Bonferroni multiple comparison test. ns, not significant.
Subsequent studies showed that CPX-351 not only did not actively promote intestinal pathology but also dampened an ongoing intestinal damage. Indeed, upon resorting to the murine model of colitis induced by the chemical irritant DSS (supplemental Figure 2A), the “7 + 3,” but not the CPX-351, treatment decreased survival (supplemental Figure 2B) and body weight (supplemental Figure 2C). Consistently, CPX-351, but not the “7 + 3” treatment, counteracted the increased epithelial permeability of colitic mice, as revealed by the reduced serum level of fluorescein isothiocyanate–dextran (supplemental Figure 2D), bacterial translocation to draining lymph nodes (supplemental Figure 2E), and attenuation of intestinal immunopathology (supplemental Figure 2F). Of interest, similar results were obtained when both drugs were administered intraperitoneally, a route of delivery associated with severe toxicity22 as revealed by acute mortality and signs of severe intestinal pathology and immune dysregulation observed after treatment with the “7 + 3” combination but not CPX-351 (supplemental Figure 2G-L). Because drugs administered intraperitoneally may undergo hepatic metabolism before reaching the systemic circulation,23 these results indicate that even in conditions of increased drug toxicity, CPX-351 may retain the ability to preserve the epithelial barrier function and prevent intestinal inflammation.
CPX-351 activates the protective AhR pathway
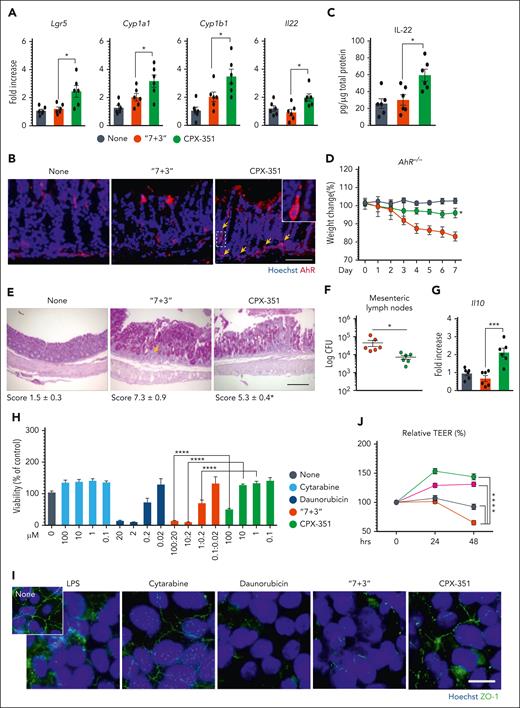
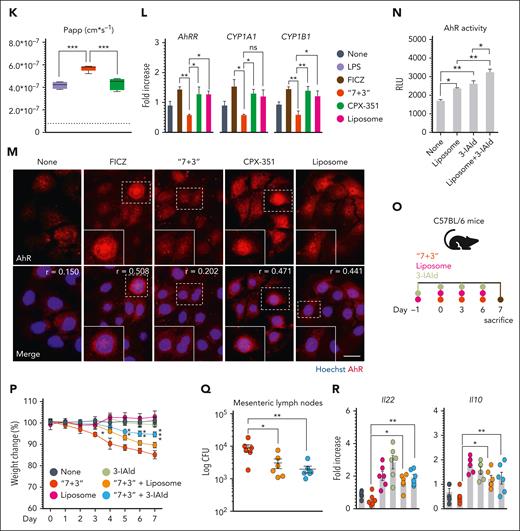
To get insight into molecular mechanisms underlying the protection afforded by CPX-351, we evaluated the expression and activation of the aryl hydrocarbon receptor (AhR), a ligand-dependent transcription factor known to protect from damage, inflammation, and dysbiosis at mucosal surfaces24,25 in vivo and in vitro in CaCo-2 cells whose permeability is regulated by AhR.26 We found that the expression of genes encoding the cytochrome P450 family 1 subfamily A member 1, Cyp1a1, Cyp1b1, the protective Il2227, the marker of intestinal cell stem,27 the leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5) (Figure 2A), and that of AhR protein (Figure 2B) was significantly promoted in the ileum and colon by CPX-351, but not the “7+3” combination, and correlated with functional activity, as revealed by the increased production of IL-22 (Figure 2C). Experiments in AhR‒/‒ mice directly proved the role of AhR in mediating the protective activity of CPX-351, as revealed by the loss of weight (Figure 2D), high histopathological score (Figure 2E), and bacterial dissemination (Figure 2F) observed upon treatment. However, the less damaging activity observed compared with the “7 + 3” treatment led us to verify whether additional mucosal protective mechanisms could be activated. Consistent with the high levels of IL-10 observed (Figure 2G) and the well-known activity of IL-10 in epithelial barrier function and immune intestinal homeostasis,28 we found that, similar to what was observed in AhR‒/‒ mice, CPX-351 promoted intestinal pathology and bacterial dissemination (supplemental Figure 3A-C) associated with high levels of AhR activity (supplemental Figure 3D) in Il10‒/‒ mice, although to a lesser degree than “7 + 3,” a finding suggesting that IL-10 is likely contributing to the protection afforded by CPX-351. In vitro, upon exposure of CaCo-2 cell monolayer to different concentrations of either drug, daunorubicin, and not cytarabine, was extremely toxic, with the toxicity being maintained in the equivalent “7 + 3” combination (Figure 2H) and associated with impaired ZO-1 protein expression (Figure 2I) and increased epithelial permeability (Figure 2J). Confirming the in vivo data, CPX-351 not only failed to induce epithelial damage but also showed protective effects against the epithelial damage induced by exposure to lipopolysaccharide (Figure 2K). Similar to in vivo, CPX-351, but not the “7 + 3” combination, activated the AhR-dependent genes (Figure 2L) by promoting its nuclear translocation (Figure 2M). Thus, similar to AhR agonists,26 CPX-351 appeared to strength the intestinal epithelial barrier function. Considering that encapsulation has been shown to enhance AhR nuclear translocation and activation,29 we assessed the activity of empty liposomes mimicking the empty liposome of CPX-35130 in vitro and in vivo. In vitro, the liposomes recapitulated the effects of CPX-351 on intestinal permeability (Figure 2J), activated AhR on CaCo-2 cells (Figure 2L and M), and, of interest, potentiated AhR activation in response to the microbial AhR ligand, indole-3-aldehyde (3-IAld), known for its mucosal protective activity31 (Figure 2N). In vivo (Figure 2O), the concomitant administration of either empty liposomes or 3-IAld attenuated the intestinal morbidity induced by the “7 + 3” treatment (Figure 2P and Q) while promoting IL-22 and IL-10 production (Figure 2R). These findings point to the contribution of the liposomes to the protection afforded by CPX-351 in vivo via the potentiation of AhR signaling in response to local microbial stimuli.
CPX-351 activates the protective AhR pathway. C57BL/6 mice were treated intravenously with the “7 + 3” combination or CPX-351 every 3 days for 3 times and assessed for (A) AhR-related genes expression in the ileum, (B) AhR immunofluorescence, and (C) IL-22 production in colon. Yellow arrows indicate AhR+ cells. AhR–/– mice were treated as above and assessed for (D) weight loss (daily measured), (E) intestinal histopathology (PAS staining) with the relative histology score, (F) bacterial translocation to mesenteric lymph nodes, and (G) Il10 gene expression in the colon. (H) Viability of CaCo-2 cells exposed to cytarabine, daunorubicin, alone, or in combination, or CPX-351 by MTT assay. (I) ZO-1 expression in differentiated CaCo-2 cells exposed as above for 24 hours. (J) Transepithelial electrical resistance (TEER) and (K) apparent permeability coefficient (Papp) of CaCo-2 cells exposed to 10 μM cytarabine, 2 μM dauno, 10 μM CPX-351, and 10 μM empty liposomes in the presence of 25 ng/mL lipopolysaccharide (LPS). The dotted line indicates untreated cells. (L) AhR-related gene expression by reverse transcription polymerase chain reaction and (M) AhR nuclear translocation by immunofluorescence in CaCo-2 cells treated as above for 4 hours (FICZ was used as control). (N) AhR activity by the luciferase assay on the reporter cell line H1L6.1c3. (O) C57BL/6 mice were concurrently treated with empty liposomes or indole-3-aldehyde (3-IAld) and the “7 + 3” combination and evaluated for (P) weight loss, (Q) bacterial translocation to mesenteric lymph nodes, and (R) Il22 and Il10 gene expression in the colon. Photographs were taken with a high-resolution microscope (Olympus BX51); original magnification ×40 (B,E; scale bars, 100 μm) and ×100 (I,M; scale bars, 20 μm). For immunofluorescence, nuclei were counterstained with Hoechst. Numbers refer to colocalization coefficients to quantify the overlap degree of AhR and Hoechst. Data are representative of 2 or 3 independent experiments. Each in vivo experiment includes 6 mice per group. Data are represented as mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; CPX-351- vs “7 + 3”–treated mice or cells; “7 + 3”- or liposome or 3-IAld vs none (untreated) cells; “7 + 3”- vs FICZ-treated cells; CPX-351 vs liposome-treated cells; liposome + 3-IAld vs 3-IAld- or liposome-treated cells; “7 + 3” + liposome or “7 + 3” + 3-IAld- vs “7+3”–treated mice. One-way or 2-way ANOVA, Bonferroni multiple comparison test.
CPX-351 activates the protective AhR pathway. C57BL/6 mice were treated intravenously with the “7 + 3” combination or CPX-351 every 3 days for 3 times and assessed for (A) AhR-related genes expression in the ileum, (B) AhR immunofluorescence, and (C) IL-22 production in colon. Yellow arrows indicate AhR+ cells. AhR–/– mice were treated as above and assessed for (D) weight loss (daily measured), (E) intestinal histopathology (PAS staining) with the relative histology score, (F) bacterial translocation to mesenteric lymph nodes, and (G) Il10 gene expression in the colon. (H) Viability of CaCo-2 cells exposed to cytarabine, daunorubicin, alone, or in combination, or CPX-351 by MTT assay. (I) ZO-1 expression in differentiated CaCo-2 cells exposed as above for 24 hours. (J) Transepithelial electrical resistance (TEER) and (K) apparent permeability coefficient (Papp) of CaCo-2 cells exposed to 10 μM cytarabine, 2 μM dauno, 10 μM CPX-351, and 10 μM empty liposomes in the presence of 25 ng/mL lipopolysaccharide (LPS). The dotted line indicates untreated cells. (L) AhR-related gene expression by reverse transcription polymerase chain reaction and (M) AhR nuclear translocation by immunofluorescence in CaCo-2 cells treated as above for 4 hours (FICZ was used as control). (N) AhR activity by the luciferase assay on the reporter cell line H1L6.1c3. (O) C57BL/6 mice were concurrently treated with empty liposomes or indole-3-aldehyde (3-IAld) and the “7 + 3” combination and evaluated for (P) weight loss, (Q) bacterial translocation to mesenteric lymph nodes, and (R) Il22 and Il10 gene expression in the colon. Photographs were taken with a high-resolution microscope (Olympus BX51); original magnification ×40 (B,E; scale bars, 100 μm) and ×100 (I,M; scale bars, 20 μm). For immunofluorescence, nuclei were counterstained with Hoechst. Numbers refer to colocalization coefficients to quantify the overlap degree of AhR and Hoechst. Data are representative of 2 or 3 independent experiments. Each in vivo experiment includes 6 mice per group. Data are represented as mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; CPX-351- vs “7 + 3”–treated mice or cells; “7 + 3”- or liposome or 3-IAld vs none (untreated) cells; “7 + 3”- vs FICZ-treated cells; CPX-351 vs liposome-treated cells; liposome + 3-IAld vs 3-IAld- or liposome-treated cells; “7 + 3” + liposome or “7 + 3” + 3-IAld- vs “7+3”–treated mice. One-way or 2-way ANOVA, Bonferroni multiple comparison test.
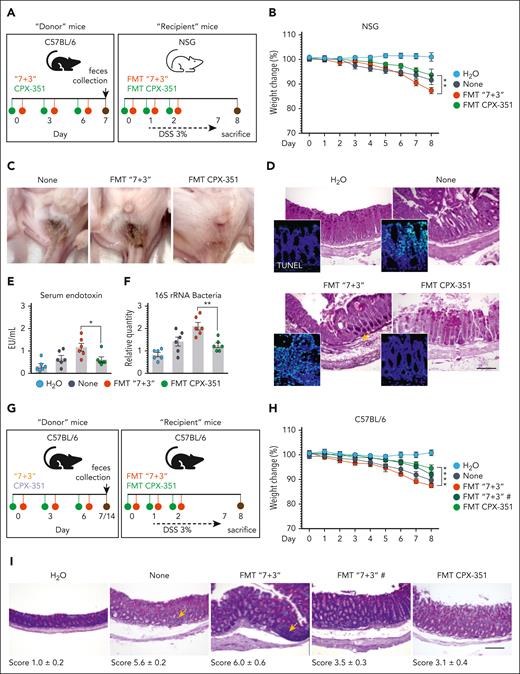
CPX-351 promotes transplantable protective microbial community
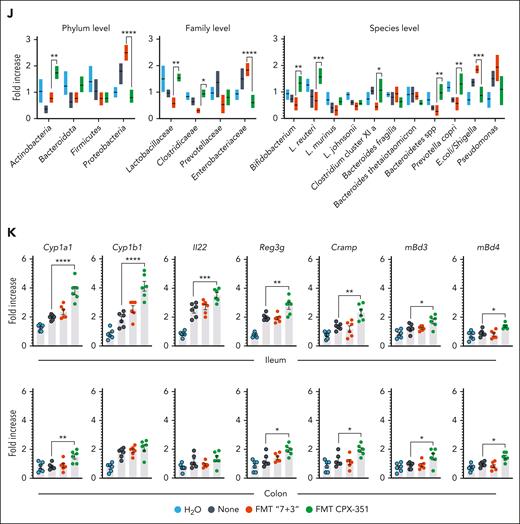
Given the potential of the AhR/IL-22 pathway to modulate the microbiome through direct and indirect effects,32,33 we assessed the contribution of intestinal microbiota to the protective activity of CPX-351 in intestinal pathology by resorting to fecal microbiota transplantation (FMT) into C57BL/6 or NSG mice with colitis (Figure 3A) and metagenomic sequencing. FMT from CPX-351–treated mice rescued weight loss (Figure 3B and H), ameliorated gross (Figure 3C) and intestinal pathology (Figure 3D and I), prevented epithelial damage (Figure 3D, insets), and decreased serum endotoxin (Figure 3E) and bacterial translocation (Figure 3F) in the dysbiotic colitic mice. In contrast, feces from “7 + 3”–treated mice consistently promoted intestinal morbidity and pathology when transplanted immediately after the treatment or a week of rest (Figure 3B-I), making it unlikely the possible contribution of residual drugs in the feces. Of interest, on assessing the microbiota composition after FMT, we have found that FMT from CPX-351-treated, but not “7 + 3”–treated, mice significantly altered the recipient microbiota composition in C57BL/6 mice, by restoring the levels of Actinobacteria (phylum and species), Bacteroidota (species), and Firmicutes (family and species). The level of Proteobacteria (phylum, family, and species) was instead significantly decreased by FMT–CPX-351 and further increased by FMT–“7 + 3” (Figure 3J). To prove that the observed recipient microbial composition reflected a functional engraftment, we also measured levels of activation of the protective AhR and defensins pathways in the ileum and colon of mice that received transplantation and found that both pathways were significantly activated upon FMT–CPX-351 but not FMT–“7 + 3” (Figure 3K). Mirroring the results obtained by targeted polymerase chain reaction (supplemental Figure 4) and 16S rRNA sequencing (see below), these preliminary evidence indicate that the engraftment of Actinobacteria, Bacteroidota, and Firmicutes upon FMT-CPX-351 is functionally successful.
CPX-351 promotes transplantable protective microbial community. Fecal microbiota transplantation in NSG (A) or C57BL/6 (G) mice with DSS-induced colitis. DSS-NSG mice received transplantation with fresh fecal pellets from C57BL/6 mice treated IV with the “7 + 3” combination or CPX-351, as in the legend to Figure 1. Mice were assessed (8 dpi) for (B) weight loss (daily measured), (C) fecal consistency, (D) colon histopathology (PAS staining) and TUNEL assay in the insets, (E) serum endotoxin, and (F) 16S rRNA bacteria expression in mesenteric lymph nodes. (G) DSS-C57BL/6 mice received transplantation with feces taken immediately or a week after the “7 + 3” treatment were evaluated for (H) weight loss and (I) colon histopathology (PAS staining) with the relative histology score. (J) Microbial composition in the feces of C57BL/6 mice after FMT from CPX-351-treated or “7 + 3”–treated mice by targeted polymerase chain reaction. (K) AhR-related and defensin genes expression by reverse transcription polymerase chain reaction in ileum and colon of C57BL/6 mice that received transplantation as above. Assays were done a week after FMT. Photographs were taken with a high-resolution microscope (Olympus BX51×); original magnification ×20 (D,I; scale bars, 200 μm). Yellow arrows indicate inflammatory foci. Data are representative of 2 or 3 independent experiments. Each in vivo experiment includes 6 mice per group. Data are represented as mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; FMT CPX-351 vs FMT “7 + 3”; none vs FMT CPX-351 and FMT “7 + 3.” One-way and 2-way ANOVA, Bonferroni multiple comparison test. None, mice treated with DSS alone. FMT “7 + 3”#, FMT with fecal pellets collected one week after the “7+3” treatment.
CPX-351 promotes transplantable protective microbial community. Fecal microbiota transplantation in NSG (A) or C57BL/6 (G) mice with DSS-induced colitis. DSS-NSG mice received transplantation with fresh fecal pellets from C57BL/6 mice treated IV with the “7 + 3” combination or CPX-351, as in the legend to Figure 1. Mice were assessed (8 dpi) for (B) weight loss (daily measured), (C) fecal consistency, (D) colon histopathology (PAS staining) and TUNEL assay in the insets, (E) serum endotoxin, and (F) 16S rRNA bacteria expression in mesenteric lymph nodes. (G) DSS-C57BL/6 mice received transplantation with feces taken immediately or a week after the “7 + 3” treatment were evaluated for (H) weight loss and (I) colon histopathology (PAS staining) with the relative histology score. (J) Microbial composition in the feces of C57BL/6 mice after FMT from CPX-351-treated or “7 + 3”–treated mice by targeted polymerase chain reaction. (K) AhR-related and defensin genes expression by reverse transcription polymerase chain reaction in ileum and colon of C57BL/6 mice that received transplantation as above. Assays were done a week after FMT. Photographs were taken with a high-resolution microscope (Olympus BX51×); original magnification ×20 (D,I; scale bars, 200 μm). Yellow arrows indicate inflammatory foci. Data are representative of 2 or 3 independent experiments. Each in vivo experiment includes 6 mice per group. Data are represented as mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; FMT CPX-351 vs FMT “7 + 3”; none vs FMT CPX-351 and FMT “7 + 3.” One-way and 2-way ANOVA, Bonferroni multiple comparison test. None, mice treated with DSS alone. FMT “7 + 3”#, FMT with fecal pellets collected one week after the “7+3” treatment.
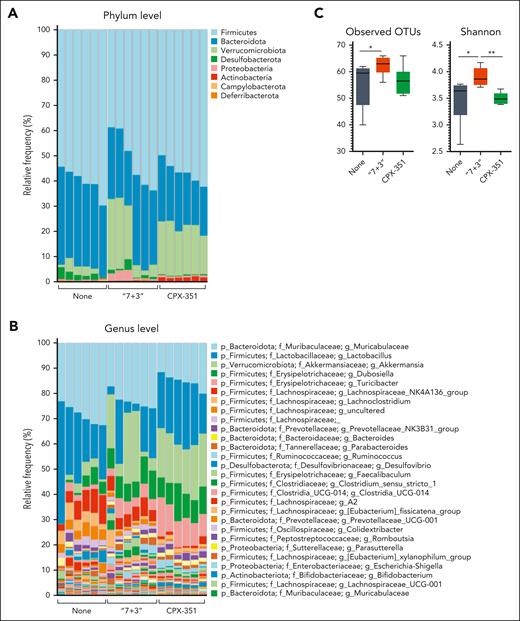
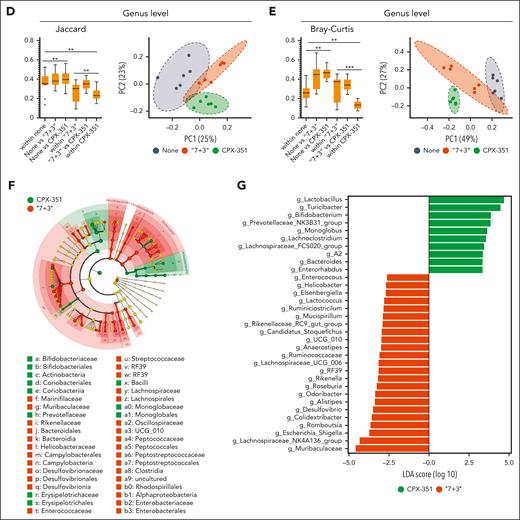
CPX-351 does not induce microbial dysbiosis
The metagenomics analysis, carried out by a customized 16S rRNA sequencing protocol amplifying the V3-V4 region and confirmed by targeted polymerase chain reaction (supplemental Figure 4), revealed that Firmicutes (mostly of the Lactobacillaceae, Erysipelotrichaceae, and Lachnospiranaceae families) and Bacteroidota (Muribaculaceae and Prevotellaceae families) represented the most abundant phyla and families in feces upon either treatment (Figure 4A and B; supplemental Figure 5A). However, differences were also observed as Actinobacteria (Bifidobacterium genus) were mostly expanded by CPX-351 (Figure 4A and B; supplemental Figure 5A) and Proteobacteria (Escherichia/Shigella genus) by the “7 + 3” treatment (Figure 4A and B; supplemental Figure 5A). Significant differences were also observed in terms of alpha diversity, being the richness and evenness, as measured by observed operational taxonomic units and Shannon indexes (Figure 4C), increased by the “7 + 3” treatment. For beta diversity, as measured by the Jaccard distance (Figure 4D) and Bray-Curtis dissimilarity indexes (Figure 4E), differences were observed in the abundance of bacteria in “7 + 3”– or CPX-351–treated mice compared with untreated animals, as clearly shown by the PCoA analysis revealing the presence of distinct clusters of significant differences between treated and untreated mice as well as between the 2 treatments (Figure 4D and E). The linear discriminant analysis effect size (LEfSe) showed the significant (P < .05) different abundances at the genus level (Figure 4F and G), and Q2-Aldex2 pipeline executed at sequence variant level (supplemental Figure 6) specified contributing taxa (P < .05). High abundance of Actinobacteria (Bifidobacterium and Enterorhabdus genera), Bacteroidota (Prevotellaceae_NK3B31_group, Bacteroides, Muribaculaceae, and Rikenella), and Firmicutes (Turicibacter, Lachnoclostridium, Lactobacillus, Lachnospiraceae.A2, Lachnospiraceae_FCS020_group, and Monoglobus genera) was observed in CPX-351–treated as opposed to “7 + 3”–treated mice in which Proteobacteria (Escherichia_Shigella genus), Campylobacterota (Helicobacter genus), Deferribacterota (Mucispirillum genus), and Desulfobacterota (Desulfovibrio genus) predominated over Firmicutes (Lachnospiranaceae NK4136 group, RF39, Ruminococcaceae, Anaerostipes, Colidextribacter, and Romboutsia) and Bacteroidota (Muribaculaceae, Odoribacter, Roseburia, Alistipes, Rikenellaceae_RC9_gut-group, and Rikenella) (Figure 4F and G). Thus, Proteobacteria, Campylobacterota, Deferribacterota, and Desulfobacterota are mostly expanded after “7 + 3” treatment and Actinobacteria after CPX-351 treatment. Moreover, different genera of Firmicutes and Bacteroidota were expanded upon each treatment. Comparative analysis with untreated mice (supplemental Figure 5B and C) confirmed the high abundance of Proteobacteria and eubiotic Actinobacteria/ Bacteriodota as microbial signatures of “7 + 3” vs CPX-351 treatment, respectively.
CPX-351 prevents microbial dysbiosis. Metagenomic analysis in C57BL/6 mice treated IV with “7 + 3” combination or CPX-351 every 3 days for 3 times. Bar plots showing bacterial composition (abundance percentage) of each sample at (A) phylum and (B) genus levels. (C) Box plots of observed features and Shannon alpha diversity indexes. Box plots of (D) Jaccard and (E) Bray-Curtis beta diversity indexes at genus level, and scatterplot of the corresponding first 2 PCoA. (F) LEfSe cladogram and (G) LDA score histogram at the genus level. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001; treated vs untreated (None) mice and CPX-351 vs “7 + 3” treatment. Kruskal-Wallis test on all and pairwise samples. LDA, linear discriminant analysis.
CPX-351 prevents microbial dysbiosis. Metagenomic analysis in C57BL/6 mice treated IV with “7 + 3” combination or CPX-351 every 3 days for 3 times. Bar plots showing bacterial composition (abundance percentage) of each sample at (A) phylum and (B) genus levels. (C) Box plots of observed features and Shannon alpha diversity indexes. Box plots of (D) Jaccard and (E) Bray-Curtis beta diversity indexes at genus level, and scatterplot of the corresponding first 2 PCoA. (F) LEfSe cladogram and (G) LDA score histogram at the genus level. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001; treated vs untreated (None) mice and CPX-351 vs “7 + 3” treatment. Kruskal-Wallis test on all and pairwise samples. LDA, linear discriminant analysis.
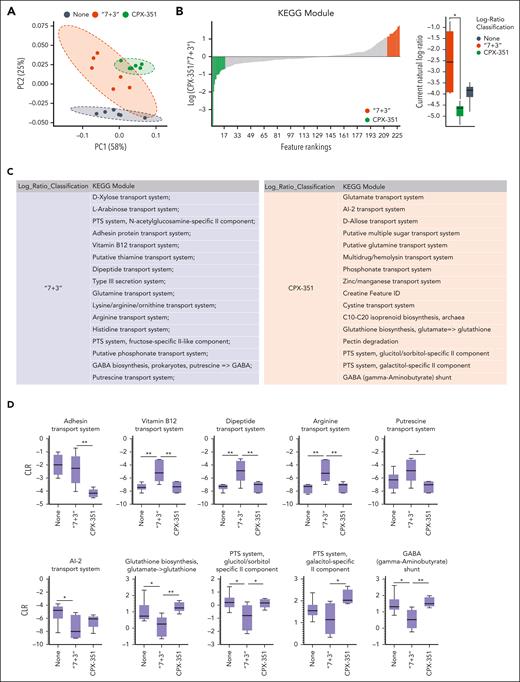
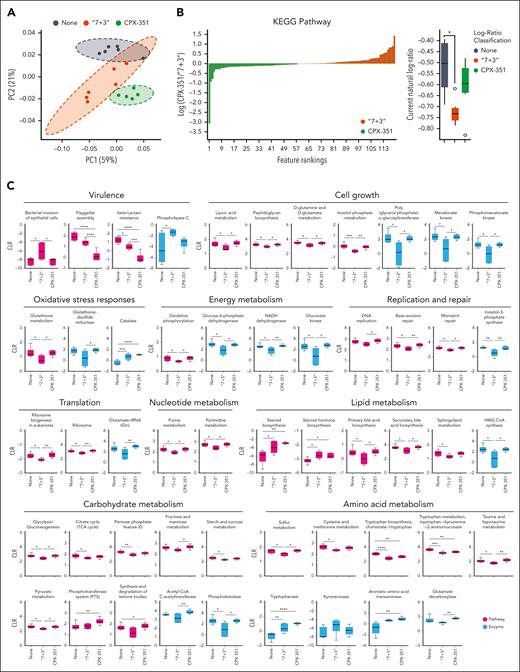
We then resorted to Phylogenetic Investigation of Communities by Reconstruction of Unobserved States 2 (PICRUSt2) and the KEGG database to determine the abundance of functional modules and pathways inferred from 16S data and to SongBird, visualized by Qurro, and quantified by centered log-ratio, to estimate differentials of functional modules. To evaluate the most contributing functions, LEfSe was applied (supplemental Tables 1-3). We found a composition of KEGG metabolic modules that clustered differently in feces from “7 + 3”–treated vs CPX-351–treated mice (Figure 5A-D). Indeed, modules associated with bacterial virulence were more abundant in “7 + 3”–treated mice, including the putrescin transport system,34 the dipeptide transport system,35 the type 3 secretion system,36 the adhesin protein transport system,37 the arginine transport system,38 and the vitamin B12 transport system,39 whereas modules associated with eubiosis, such as the autoinducer-2 molecule mediating the quorum sensing signaling in the bacterial kingdom,40 the gamma-aminobutyric acid shunt modules, and the glutathione biosynthesis, key players in mitigation of reactive oxygen species production during stress41 and the major carbohydrate transport system, phosphotransferase system, regulating a variety of metabolic processes,42 were more expanded in CPX-351-treated mice. In terms of KEGG pathways and related enzymes, visualized by PCoA, Qurro, and quantified by centered log-ratio (Figure 6A-C and supplemental Figure 7), the features' abundances confirmed the high expression of pathways associated with bacterial virulence (bacterial invasion and phospholipase c activity) and low expression of pathways associated with cell growth (cell wall and membrane biosynthesis, inositol phosphate metabolism, and the mevalonate pathway); oxidative stress response (glutathione metabolism); energy metabolism (oxidative respiration and nicotinamide adenine dinucleotide dehydrogenase); replication and repair (mismatch repair and base excision repair); nucleotide metabolism (purine and pyrimidine metabolism) and translation (ribosome biogenesis) upon the “7 + 3” treatment. In terms of specific metabolisms, the carbohydrate pathways (glycolysis, TCA cycle, gluconeogenesis, pyruvate metabolism, phosphoketolase, and starch and sucrose metabolism), the lipid pathways (primary and secondary bile acid biosynthesis, sphingolipid metabolism, and the 3-hydroxy-3-methylglutaryl coenzyme A synthesis), and the amino acid pathways (cysteine, methionine and sulfur metabolism, tryptophan (Trp) biosynthesis and metabolism, and taurine and hypotaurine metabolism) were all less represented in the microbiota of the “7 + 3”–treated mice. At variance with what was observed in these mice, the features' abundances in feces from CPX-351–treated mice included a lower expression of the virulence pathways and a rescued expression of pathways related to cell growth, oxidative stress response (worth mentioning the increased catalase activity), oxidative respiration, replication, repair, nucleotide metabolism, and translation. A similar rescued expression to normal levels was also observed for the carbohydrate pathways, including the pyruvate metabolism, phosphotransferase system, the ketone bodies synthesis and degradation, the acetyl-CoA C-acetyltransferase enzyme (EC 2.3.1.9), the lipid pathways, and the amino acid pathways. For Trp, in particular, the higher tryptophanase (EC: 4.1.99.1) and the aromatic amino acid transaminase (EC 2.6.1.57), but not kynureninase (EC 3.7.1.3), activity observed in CPX-351–treated mice suggests that a microbial, rather than a host, degradation pathway is occurring. Together with feature’s abundant differences in other metabolic pathways also observed between the 2 treatments (supplemental Figure 7), the overall data point to the occurrence of a dysbiotic microbial phenotype upon “7 + 3” treatment, characterized by increased microbial virulence, decreased aerobic respiration, and deregulated metabolic pathways, all known to be violated by anthracycline.43 CPX-351, in contrast, protected from dysbiosis, with most of the feature’s abundance expressed at the levels similar to those of untreated mice and even at the higher levels, such as the ketone body metabolism and the related acetyl-CoA C-acetyltransferase enzyme, the tryptophanase and aromatic amino acid transaminase enzymatic activities.
CPX-351 promotes the abundance of eubiotic microbial modules. (A) PCoA of predicated functions and (B) SongBird differential analysis of relevant KEGG modules abundance with corresponding log-ratio rankings visualized with Qurro in mice treated IV as indicated. The CPX-351/“7 + 3” log-ratios of top 15 differential modules were selected to generate a box-whisker plot.18 (C) Table of KEGG Modules and (D) box and-whisker plots of relevant metabolic functions by PICRUSt2. ∗P < .05; ∗∗P < .01; CPX-351- vs “7 + 3”-treated mice, “7 + 3” vs none (untreated) mice. One-way ANOVA; Bonferroni multiple comparison test. Kruskal-Wallis test on all and pairwise samples.
CPX-351 promotes the abundance of eubiotic microbial modules. (A) PCoA of predicated functions and (B) SongBird differential analysis of relevant KEGG modules abundance with corresponding log-ratio rankings visualized with Qurro in mice treated IV as indicated. The CPX-351/“7 + 3” log-ratios of top 15 differential modules were selected to generate a box-whisker plot.18 (C) Table of KEGG Modules and (D) box and-whisker plots of relevant metabolic functions by PICRUSt2. ∗P < .05; ∗∗P < .01; CPX-351- vs “7 + 3”-treated mice, “7 + 3” vs none (untreated) mice. One-way ANOVA; Bonferroni multiple comparison test. Kruskal-Wallis test on all and pairwise samples.
CPX-351 promotes the abundance of eubiotic microbial pathways. (A) PCoA of predicated functions and (B) SongBird differential analysis of relevant KEGG pathways abundance with corresponding log-ratio rankings visualized with Qurro in mice treated IV as indicated. The CPX-351/“7 + 3” log-ratios of differential modules were selected to generate a box-whisker plot.18 (C) Box-and-whisker plots of relevant metabolic functions by PICRUSt2 analysis. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001; treated vs untreated (None) mice and CPX-351 vs “7 + 3” treatment. One-way ANOVA, Tukey multiple comparison test. Kruskal-Wallis test on all and pairwise samples.
CPX-351 promotes the abundance of eubiotic microbial pathways. (A) PCoA of predicated functions and (B) SongBird differential analysis of relevant KEGG pathways abundance with corresponding log-ratio rankings visualized with Qurro in mice treated IV as indicated. The CPX-351/“7 + 3” log-ratios of differential modules were selected to generate a box-whisker plot.18 (C) Box-and-whisker plots of relevant metabolic functions by PICRUSt2 analysis. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001; treated vs untreated (None) mice and CPX-351 vs “7 + 3” treatment. One-way ANOVA, Tukey multiple comparison test. Kruskal-Wallis test on all and pairwise samples.
Microbial metabolites and colonization resistance to fungi are promoted by CPX-351
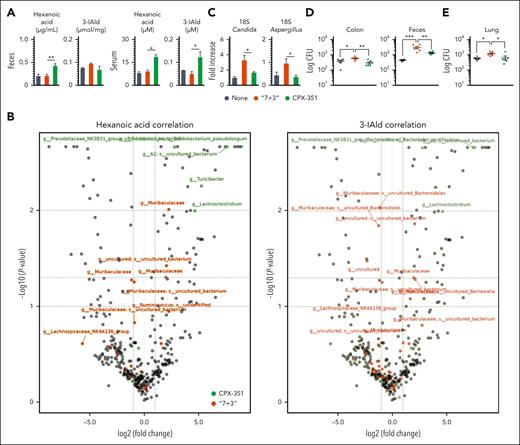
The observed microbial metabolic activities prompted us to evaluate the levels of short-chain fatty acids, medium-chain fatty acids (MCFA), and indoles in feces and sera of treated mice. Of 23 metabolites assessed by targeted metabolomics, we found higher levels of the 6-carbon chain, the hexanoic acid (also named caproic acid), in both serum and feces after CPX-351 rather than “7 + 3” treatment (Figure 7A), a finding consistent with the increased expression of the acetyl-CoA C-acetyltranferase enzyme (Figure 6C), involved in the biosynthesis of hexanoic acid in anaerobes.44 Although the levels of the acetic, propionic, butyric, and valeric short-chain fatty acids were not increased by either treatment (supplemental Figure 8A), the isobutyric and isovaleric acids, known for their contribution to inflammation,45 were higher in the feces of the “7 + 3”–treated than CPX-351–treated mice (supplemental Figure 8A). In the case of Trp metabolites, most host-derived or microbial-derived metabolites were either below the detection levels or not different in either serum or feces after either treatment (supplemental Figure 8B), with the exception of host-derived kynurenines increased in the serum by “7 + 3” and serotonin by CPX-351 (supplemental Figure 8B). Of great interest, the microbial-derived 3-IAld that attenuated the intestinal pathology inflicted by the “7 + 3” treatment (Figure 2P and Q) was significantly increased in the serum of CPX-351–treated mice (Figure 7A), consistent with the increased expression of aromatic-amino acid transaminase enzyme (Figure 6C) involved in the biosynthesis of 3-IAld in Lactobacillus reuteri46 from both Trp and the salvaging indole pathway. The Spearman correlation analysis between the fecal microbiota at the sequence variants level and metabolome revealed that the proportions of Bifidobacterium (r = 0.71; P = .1), Lachnospiraceae.A2 (r = 0.88, P = .01), Turicibacter (r = 0.37, P = .4), and Lachnoclostridium (r = 0.88; P = .01) were positively correlated with the concentration of hexanoic acid while the proportions of Bacteroides (r = 0.4; P = .4) and Lactobacillus reuteri (r = 0.2; P = .4) were positive correlated with the levels of 3-IAld in CPX-351–treated mice. The proportion of Prevotellaceae-NK3B31 group and Lachnospiraceae.A2 were positively correlated with both the hexanoic acid (r = 0.88; P = .01) and 3-IAld (r = 0.55; P = .29) concentrations (Figure 7B), consistent with the ability of either sequence variant to produce MCFA47,48 and to have tryptophanase activity.49,50 The well-known antifungal activity of both the hexanoic acid51 and 3-IAld46 led us to assess whether the colonization resistance to fungi was increased by CPX-351. Both Candida and Aspergillus colonizations were significantly increased in the feces upon treatment with “7 + 3” but not CPX-351, as revealed by 18S rRNA sequencing (Figure 7C), and accordingly, antifungal resistance was significantly impaired by treatment with “7 + 3” and not CPX-351 in mice infected with either Candidaalbicans or Aspergillus fumigatus (Figure 7D and E). Together, these data suggest that CPX-351 induces a microbial landscape characterized by the relative abundance of bacterial species producing metabolites known to affect epithelial barrier function and colonization resistance to fungi.
Production of metabolites and colonization resistance to fungi are promoted by CPX-351. (A) Levels of hexanoic acid and 3-IAld in the feces and serum of “7 + 3”- or CPX-351–treated mice. (B) Volcano plots showing the Spearman correlation analysis between the fecal microbiota at the sequence variant level and metabolites, according to the color legend. In the volcano plot, the difference between groups, expressed as logarithmic fold-change, is plotted vs the adjusted P value (Wilcoxon) of differential abundance evaluated with Q2-Aldex2. Horizontal dashed lines represent P = .1; P = .05; and P = .01. Vertical dashed lines represent fold change = 2. Colored taxa are the 30% with the strongest positive correlation values among those genera significantly associated by LEfSe (P < .05) with a treatment. Detection of fungi in the feces (C) by 18S RNA and (D) colony forming units in the feces and colon of Candida albicans–infected mice and (E) lung of Aspergillus fumigatus–infected mice treated with “7 + 3” combination or CPX-351 at 2 days after infection. Data are representative of 2 or 3 independent experiments. Each in vivo experiment includes 6 mice per group. Data are represented as mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; treated vs untreated (None) mice, CPX-351 vs “7 + 3” treatment. One-way ANOVA, Bonferroni multiple comparison test.
Production of metabolites and colonization resistance to fungi are promoted by CPX-351. (A) Levels of hexanoic acid and 3-IAld in the feces and serum of “7 + 3”- or CPX-351–treated mice. (B) Volcano plots showing the Spearman correlation analysis between the fecal microbiota at the sequence variant level and metabolites, according to the color legend. In the volcano plot, the difference between groups, expressed as logarithmic fold-change, is plotted vs the adjusted P value (Wilcoxon) of differential abundance evaluated with Q2-Aldex2. Horizontal dashed lines represent P = .1; P = .05; and P = .01. Vertical dashed lines represent fold change = 2. Colored taxa are the 30% with the strongest positive correlation values among those genera significantly associated by LEfSe (P < .05) with a treatment. Detection of fungi in the feces (C) by 18S RNA and (D) colony forming units in the feces and colon of Candida albicans–infected mice and (E) lung of Aspergillus fumigatus–infected mice treated with “7 + 3” combination or CPX-351 at 2 days after infection. Data are representative of 2 or 3 independent experiments. Each in vivo experiment includes 6 mice per group. Data are represented as mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; treated vs untreated (None) mice, CPX-351 vs “7 + 3” treatment. One-way ANOVA, Bonferroni multiple comparison test.
Discussion
This study shows that, compared with “7 + 3” combination, CPX-351 has additional important features that are relevant in AML, namely the maintenance of a functional mucosal barrier and a functional microbiota. A dysbiotic phenotype, associated with an abnormal expansion of Proteobacteria, a microbial signature of intestinal inflammation and a compromised ability to maintain a balanced gut microbial community observed in humans52 and patients with AML,21,53 was induced by treatment with “7 + 3” combination along with the expansion of other phyla associated with gut and systemic inflammation, such as Campylobacterota,54 Deferribacterota,55 and Desulfobacterota.56 Not only were those phyla not expanded by CPX-351 but also eubiotic commensals, such as Bifidobacterium57, Prevotellaceae,58 and Bacteroides,59 were expanded. Functionally, these different microbial compositions correlated with the expression of functions related to microbial virulence and deregulated metabolic activity after the “7 + 3” treatment and with microbial eubiosis, such as oxidative respiration, genomic stability via the DNA damage repair,60 and restoration of salutary metabolic activities after CPX-351 treatment. Therefore, CPX-351 seems to be able to counteract the Bacteroides and Bifidobacterium loss, the genomic instability, and defective metabolic activity described in induction chemotherapy.21,53 Indeed, the expansion was associated with the production of salutary metabolites, such as the hexanoic acid and 3-IAld, to which the expanded Bacteroidota (Bacteroides and Prevotellaceae-NK3B31 group) and Bifidobacterium contributed. Both MCFA, such as the octanoic acid (caprilic acid) and the decanoid acid (capric acid),61,62 and 3-IAld63 are well-known for their ability to support the integrity of the intestinal mucosal barrier function and to provide microbial homeostasis,20,64 including an activity on fungi.46,65 Accordingly, we found that colonization resistance to fungi was greatly promoted by CPX-351 and impaired by the “7 + 3” treatment, a finding suggesting that the beneficial effect CPX-351 may include an ability to restore the impaired colonization resistance in patients with AML, during which a defective production of both indole, an early sign of neutropenic fever11 and graft-versus-host disease,66 and hexanoic acid21 have both been observed. In this regard, it is also of interest that hexanoic acid was found to reduce oxygen radical production in neutrophils,67 an activity that may limit the neutrophil-dependent pathology in graft-versus-host disease68 and is consistent with the low hexanoic acid levels in recurrent Crohn disease.69
Because the microbiota and the intestinal epithelial barrier are reciprocally regulated, the beneficial activity of CPX-351 may occur at both levels. Different from cytarabine, anthracyclines are known to increase the mucosal permeability and the ensuing infectious complications,2,70 to which the direct inhibition of the growth of Gram-positive, Gram-negative43 and anaerobic71 bacteria likely contribute. This points to unique properties CPX-351 may have in providing mucosa immune homeostasis in the gut. We found that CPX-351 uniquely activated the host protective AhR pathway in the murine gut and human intestinal cells in vitro, an activity that is consistent with both the epithelial barrier protection and strengthening, the decreased inflammation, and the occurrence of immune tolerance. The liposome is a likely candidate for this activity, given the well-known ability of liposomes to interact with and affect the activity of immune cells72 as well as of microbes.73 We have found that an empty liposome, similar in composition to Vyxeos, was able to activate AhR in intestinal epithelial cells to an extent similar to that of CPX-351, whereas neither drug in the “7 + 3” combination did so. Of great interest, we found that empty liposomes also increased AhR activation in response to 3-IAld, a finding suggesting that liposomes may combine with endogenous ligands for optimal AhR activation in the gut. Because AhR,74 IL-22,75 and IL-1028 all contribute to the maintenance of a salutary gut microbiota, providing microbial eubiosis via the activation of host AhR/IL-22/IL-10 pathway is a plausible mechanism of action of CPX-351. This appeared to be the case because, similar to 3-IAld, empty liposomes administration mitigated the “7 + 3”–induced immunopathology while inducing IL-22 and IL-10 production. Thus, liposomes, by mitigating the toxic effect of daunorubicin in the intestine, may break down the escalating vicious circle between barrier dysfunction and dysbiosis, an activity consistent with the ability of empty liposomes to attenuate immunopathology in vivo.76 Ultimately, the superior t1/2 of CPX-351 in tissues, including the small intestine and the lung77 and its uptake by nonmalignant cells,78 suggests a role of liposomes in vivo. Alternatively, similar to a coculture cell model mimicking gut inflammation,79 where the inflammatory damage inflicted by the “7 + 3” combination on epithelial cells occurred either directly or through the damaged myeloid cells (supplemental Figure 9A and B), AhR activation could result from local intercellular cross talk. Whatever the case, although the elucidation of receptors and pathways contributing to the liposome-mediated effects in vitro and in vivo is ongoing, the ability of the AhR-dependent manganese superoxide dismutase80 to attenuate cardiotoxicity,81 may explain the less cardiotoxicity of CPX-351 compared with daunorubicin.82
Altogether, this study provides mechanistic explanations of the superior safety profile of CPX-351 in patients with AML, which include strengthening the intestinal epithelial, taming intestinal inflammation, and preventing microbial dysbiosis. The study also highlights how the alpha diversity metric alone, without the signature of specific bacterial abundances, may not be informative of microbial eubiosis vs dysbiosis. The results have clearly showed that a more diverse microbiota in the “7 + 3” treatment was associated with pathology. Of course, the inherent limitations of our preclinical models of mucositis are the main barriers to the rapid translation of this knowledge into precision medicine. Future prospective studies in humans that assess the fecal microbiota after intensive chemotherapy are needed to document the role of the major covariate, that is, the concomitant antibiotic administration, and to define the impact of nutrition and additional therapies on the CPX-351-host-microbiota triade. This will help to define prognostic algorithms and novel end points of therapy, as well as optimize antibiotic-descalation strategies, considering that carbapenem increases infection risk12 as opposed to the less microbiota-disruptive levofloxacin,83 and future combination therapy with CPX-351.
Acknowledgment
This study was supported in part by research funding from Jazz Pharmaceuticals, IST_11389.
Authorship
Contribution: G.R., L.F., L.P., and L.R. contributed to conceptualization; G.R., C.S., M. Pariano, M.F., N.P., and P.M. contributed to methodology; M. Puccetti contributed to indole-3-aldehyde study; G.P. and C.D.S. contributed to mass spectrometry; N.P. and M.F. contributed to liposome preparation; E.N. contributed to metagenomics analysis; G.R. and L.R. contributed to writing, reviewing. and editing the manuscript; E.G. contributed to imaging; V.O. contributed to graphical editing; L.R. and L.P. provided supervision and acquired funding; and all authors have read and agreed to the published version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Luigina Romani, Department of Medicine and Surgery, University of Perugia, P.le Lucio Severi 1, 06132 Perugia, Italy; email: luigina.romani@unipg.it.
References
Author notes
G.R., E.N., and C.S. are joint first authors.
L.P. and L.R. are joint senior authors.
The data sets generated and analyzed during this study are available at BioProject ID PRJNA979985 (https://dataview.ncbi.nlm.nih.gov/object/PRJNA979985?reviewer=jqqjj0impj6h4n9il82q0dmq3c).
Data are available upon reasonable request from the corresponding author, Luigina Romani (luigina.romani@unipg.it).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal