Visual Abstract
Over the past 10 years, there has been a marked increase in recognition of the interplay between the intestinal microbiome and the hematopoietic system. Despite their apparent distance in the body, a large literature now supports the relevance of the normal intestinal microbiota to steady-state blood production, affecting both hematopoietic stem and progenitor cells as well as differentiated immune cells. Microbial metabolites enter the circulation where they can trigger cytokine signaling that influences hematopoiesis. Furthermore, the state of the microbiome is now recognized to affect outcomes from hematopoietic stem cell transplant, immunotherapy, and cellular therapies for hematologic malignancies. Here we review the mechanisms by which microbiotas influence hematopoiesis in development and adulthood as well as the avenues by which microbiotas are thought to impact stem cell transplant engraftment, graft-versus-host disease, and efficacy of cell and immunotherapies. We highlight areas of future research that may lead to reduced adverse effects of antibiotic use and improved outcomes for patients with hematologic conditions.
The microbiome in human health and hematologic disease
The human gut microbiome is the collection of intestinal microorganisms that establishes a commensal relationship with the host, affecting diverse biologic functions such as nutrient uptake and metabolism, drug metabolism, and regulation of the immune system. Alterations in the gut microbiome have been linked to a wide array of pathologies including blood diseases and cancer.1-3 Manipulation of the gut microbiota has been studied in preclinical and clinical studies in the setting of inflammatory diseases such as inflammatory bowel disease, metabolic syndrome, and coronary artery disease.4,5
Initial evidence that the microbiota influences the hematologic system stemmed from observations that blood counts are abnormal in germ-free (GF) mice.3 Local microbially-driven immunomodulation in the gut has now been well described, but more recent studies, including our own,6-8 have focused on how the microbiome influences distant processes including hematopoietic function.9 In this review, we summarize the relationship between the intestinal microbiota and steady-state hematopoiesis. Further, we discuss how disruption of the microbiome influences hematopoiesis and treatment outcomes of hematopoietic stem cell transplantation, immunotherapy, and cellular therapy for hematologic malignancies.
Hematologic complications of prolonged antibiotics
Early evidence for a connection between the gut microbiome and hematopoiesis arose from the observation that cytopenia or low peripheral blood cells counts are a major complication of antibiotic use. A retrospective study showed that neutropenia, rash, and nephrotoxicity were the most prevalent side effects, occurring in one-third of patients receiving outpatient parenteral antimicrobial therapy.10 A recent retrospective observational study of hospitalized children showed that antibiotics were associated with adverse hematologic complications such as leukopenia (29% of hematologic adverse events), neutropenia (24%), eosinophilia (21%), anemia (18%), and thrombocytopenia (8%).11 Previous studies have shown that as many as 15% of people who receive antibiotics for 2 weeks or longer experience neutropenia and other cytopenia that are associated with poor outcomes and increased costs.12
Prolonged antibiotics are utilized worldwide to treat life-threatening bacterial infections and for prophylaxis after surgical and dental procedures, with >200 million courses of antibiotics were prescribed in the year 2022 in US alone. A global study reviewed antibiotic usage and consumption data from 204 countries and found a staggering rise of 46% in antibiotic consumption rates between 2000 and 2018.13 Thus, widespread antibiotic use translates into a significant burden of detrimental hematologic side effects, including anemia, neutropenia, and pancytopenia.
Understanding which antibiotic classes are associated with cytopenia may provide a clue to the underlying mechanism. Many reports of antibiotic-associated cytopenia have focused on β-lactam antibiotics.14 A retrospective cohort study of patients seen in the infectious diseases clinic of a tertiary referral children's hospital from 2009 to 2011 found β-lactams to be the most common causative agent of blood-related complications.15 Despite these reports, cytopenias have been reported with a wide range of antibiotics. A systemic database review of case reports from 1968 to 2020 found that high-dose treatment with glycopeptides (eg, vancomycin) over long periods was associated with increased risk of acute antibiotic-induced neutropenia, which was improved upon the use of granulocyte colony-stimulating growth factors (G-CSFs).16 Curtis et al reported that fluoroquinolones were frequent causes of drug-induced neutropenia.17 In our own recent analysis of a series of pediatric patients who developed neutropenia while on antibiotic courses of 2 weeks or more, β-lactams, cephalosporins, lincosamides, glycopeptides, and macrolides were all represented without a clear association between antibiotic class and risk of neutropenia (J. Fernandez Sanchez, R. Rodgers, A. Maknojia, et al. unpublished data, March 2024). However, our study was not powered to detect such an association and most studies of antibiotic adverse effects have not been designed to address this question.
Several prior studies have suggested immune mechanisms for antibiotic-induced neutropenia, also known as idiopathic drug-induced neutropenia (IDIN). Evidence for an immune mediated mechanism include some reports that HLA type is associated with IDIN or the possibility that antibiotics created autoantigens. However, the purported autoantibodies have been difficult to detect. Analysis of serum collected during the period 2012 to 2016 from patients with suspicion of IDIN found no drug-dependent antibodies, suggesting more of an indirect effect.17
In our studies of antibiotic-associated cytopenia in a mouse model, direct incubation of hematopoietic progenitors with antibiotics did not impair their colony forming capacity, suggesting a lack of direct toxicity. However, 2-week oral administration of a broad-spectrum antibiotic cocktail consistently resulted in bone marrow (BM) suppression. Importantly, the frequency of hematopoietic progenitors in GF mice phenocopied those of antibiotic-treated mice and was not further suppressed upon administration of antibiotics.6 These data suggest that antibiotic suppression of the microbiota plays an important mechanistic role in antibiotic-associated cytopenias. We discuss the mechanisms underlying such interactions below.
Mechanisms of microbiome-sustained hematopoiesis
There has been growing appreciation of the role of microbiome in regulating steady-state hematopoiesis. The mammalian gastrointestinal tract harbors a complex community of microbes including fungi, bacteria, viruses, and archaea that are critical to human health and well-being. This commensal microbial community provides benefits to its host by regulating various aspects of host physiology such digestion, host behavior, detoxification, energy metabolism, protection against opportunistic pathogens, and regulation of immune system. Indeed, the gut barrier, which acts as a physical and immunological defense against luminal microorganisms, viruses, food antigens, and environmental toxins, is selectively permeable to allow for translocation of select microbial metabolites from the intestinal lumen into the circulation where they regulate production and function of various immune cell types.18
Mouse models have been useful for deciphering the dynamic interplay between the gut microbiome and host factors.19 Although GF mouse models are a gold standard for studying microbiome-host immune crosstalk, an inexpensive alternative is the use of antibiotics to modify the gut microbiome. Both GF and antibiotic-treated mice exhibit hematologic defects including abnormal splenic and BM myeloid cell counts, suppressed hematopoietic stem and progenitor cells (HSPCs), and aberrant T-cell responses.9,19 To address differences between mouse and human microbiotas, humanizing GF mice with human microbiota or defined microbial communities can serve as a great tool for assessing causal relationships between the microbiome and human diseases.20
Microbiota-driven immune signaling pathways and their role in hematopoietic maintenance
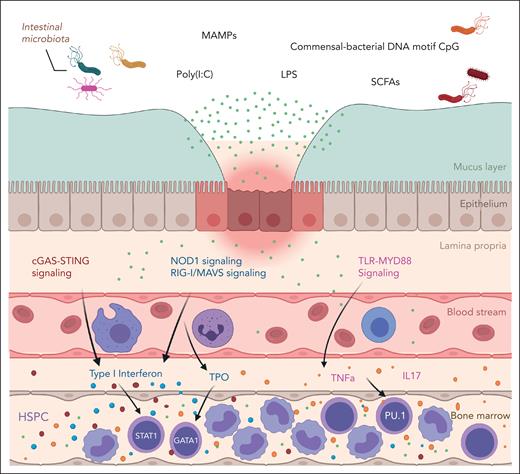
In a recent study, we used mouse models to demonstrate that broad-spectrum antibiotic treatment ablates the intestinal microbiome, reduces type I interferon (IFN-I) and downstream STAT1 signaling, and suppresses the number of hematopoietic progenitors and granulocytes in the murine BM.8 Conditional knock out and reciprocal transplants performed using Stat1-floxed mice revealed that microbiome-driven STAT1 signaling is required in the hematopoietic compartment to support normal blood production. Further, we demonstrated that oral supplementation with the bacterial cell wall component nucleotide binding oligomerization domain 1 (NOD1) ligand (NOD1L) was sufficient to restore granulocyte counts in antibiotic-treated mice. Altogether, these data support a model in which microbial products translocate from the intestine to support signaling in the BM to promote steady-state hematopoiesis (Figure 1).
The microbiota signals to the BM to support hematopoiesis. The intestinal microbiome produces metabolites such as peptidoglycans, SCFA, LPS, and nucleic acids that can be detected by signal transducers such as TLR, STING/GAS, and NOD1. These, in turn, generate type I interferon, TPO, TNFa, and IL-17 signaling, which have been shown to support hematopoiesis via STAT1 and other downstream pathways in the BM. cGAS, cyclic GMP-AMP synthase; LPS, lipopolysaccharide; MAMPs, microbe associated molecular patterns; STING, stimulator of interferon response; TNFa, tumor necrosis factor alpha; TPO, thrombopoietin.
The microbiota signals to the BM to support hematopoiesis. The intestinal microbiome produces metabolites such as peptidoglycans, SCFA, LPS, and nucleic acids that can be detected by signal transducers such as TLR, STING/GAS, and NOD1. These, in turn, generate type I interferon, TPO, TNFa, and IL-17 signaling, which have been shown to support hematopoiesis via STAT1 and other downstream pathways in the BM. cGAS, cyclic GMP-AMP synthase; LPS, lipopolysaccharide; MAMPs, microbe associated molecular patterns; STING, stimulator of interferon response; TNFa, tumor necrosis factor alpha; TPO, thrombopoietin.
Consistent with our findings, several studies have shown that microbiota-derived components can translocate across the intestinal epithelium and are recognized by pattern recognition receptors to affect hematopoiesis. Bacterial peptidoglycan is a known circulating activator of NOD1 in macrophages, which can, in turn, trigger production of neutrophil chemoattractant CXCL1 to promote inflammation.21 Oral administration of NOD1L restores normal hematopoiesis in GF mice.22 Similarly, bacterial cell wall products can promote G-CSF production and myelopoiesis via endogenous phospholipase C β-3 (PLCβ-3) activation in endothelial cells.23 Lee et al24 reported that microbiota-derived lactate can travel to BM via systemic circulation and activate stem cell factor (SCF) expression by LepR+ cells to promote hematopoiesis in a Gpr81-dependent manner. This pathway regulates hematopoietic stem cell (HSC) self-renewal and differentiation as well as BM recovery after irradiation or busulfan stress.24 At the embryonic stage of development, NOD1-RIPK2 signaling, another microbial product sensor, has been reported to mediate HSPC specification and differentiation via NF-κB activation.25 Furthermore, a recent study showed that colonization of GF zebrafish with the commensal strain Aeromonas dhakensis affected basal inflammatory cytokine signaling and restored HSPC development both in caudal hematopoietic tissue and kidney.26 Thus, circulating microbial metabolites can act through a variety of signaling pathways in BM niche cells such as stroma and endothelial cells to support hematopoiesis (Figure 1).
Microbial sensing is also likely to occur directly in hematopoietic progenitors via toll-like receptors (TLRs).27 Primitive hematopoietic progenitor cells abundantly expressed TLR2 and TLR4-MD-2. Ligation of TLRs in culture drives cell cycle entry. Furthermore, activation of TLR on myeloid progenitors drives the emergence of monocytes and/or macrophages in the absence of exogenous growth and differentiation factors, suggesting that undifferentiated progenitor cells can use microbial components as cues for hematopoietic cell development.28 Jackson et al reported that persistent stimulation of TLR7 at epithelial surfaces drives myelopoiesis via the release and differentiation of immature premonocytes in the BM.29 Similarly, De Luca et al reported that activation of TLR1/2 on human HSCs via PAM3CSK4 treatment profoundly represses B-cell emergence while promoting differentiation of common myeloid progenitors and, to a lesser degree, granulocyte-monocyte progenitors, and megakaryocyte-erythroid progenitors.30 PAM3CSK4-mediated priming of HSCs is associated with upregulated expression of GATA-1, PU.1, and C/EBPα, all known to promote myelopoiesis. Augmented TLR2 signaling via PAM3CSK4 exposure, results in expansion of BM and spleen HSPCs associated with induction of tumor necrosis factor-α (TNFα).31
Other bacterial sensors such as the NOD2, RIG-I, and stimulator of interferon response (STING) pathways have also been reported to influence hematopoiesis cell autonomously. NOD2 signaling pathway is sufficient to drive the expression of TNF-α, granulocyte monocyte colony stimulating factor (GM-CSF), CD11c, CD14, CD206, and PU.1 in human CD34+ cells resulting in myeloid differentiation.32 Disruption of downstream IFN-I signaling through the intracellular viral sensing receptor RIG-I significantly impaired the development of hematopoietic precursors without impacting endothelial precursors and angiogenesis.33 Furthermore, RIG-I morphant embryos displayed a remarkable reduction in the expression of lineage-specific markers such as pu.1/spi1, lcp1/l-plastin, and mpo/mpx, along with erythroid lineage marker gata-1.33 Similarly, a recent study showed that endogenous repetitive element RNAs, which can include endogenous retroviruses, can activate RIG-I-like receptors during embryonic development to regulate HSPC formation via ubiquitination and activation of downstream mediator TRAF6.34 Further, the bacteria-derived molecule c-di-guanosine monophosphate (GMP) activates innate immune sensor STING (an inducer of IFN-I) to promote HSC mobilization and entry into the cell cycle and increase multipotent progenitor expansion.35
There is also growing evidence that nucleic acids derived from the microbiota can be sensed by intracellular receptors. Gutierrez-Merino et al reported that pathogen associated molecular patterns derived from Pediococcus pentosaceus, Akkermansia muciniphila, and Lactobacillus plantarum can be sensed by nucleic acid sensors, STING and RIG-I, triggering endogenous IFN-I production by human primary immune cells.36,37 Similarly, membrane vesicles containing bacterial DNA derived from several bacterial phyla including Firmicutes, Bacteroidota, Proteobacteria, and Actinobacteriota can mediate systemic IFN-I priming via the activation of cyclic GMP-AMP synthase (cGAS)-STING pathway.38 Collectively, these data show microbial signals may activate IFN-I through NOD2, RIG-I, or STING pathways and are consistent with our own studies pointing toward the importance of IFN-I in hematopoiesis.39-41 Altogether, signaling pathways known to impact hematopoiesis are summarized in Table 1.
Microbiota-driven immune signaling pathways and their effects on the hematopoietic system
| Microbiome-driven signaling pathway . | Effects on hematopoietic system . | Reference . |
|---|---|---|
| NOD1 signaling | Promotes steady-state hematopoiesis indirectly through the induction of cytokines by mesenchymal stromal cells | 42 |
| RAC1-NOD1-RIPK2-NF-κB axis initiates inflammatory signaling in the HE, resulting in HSPC specification | 25 | |
| NOD1 signaling in hematopoietic compartment regulates proinflammatory macrophage polarization and neutrophil infiltration in adipose tissue | 22 | |
| TLR-MYD88 signaling | Bacterial recognition during infection promotes the G-CSF secretion from endothelial cells and monocytes, leading to the acceleration of granulopoiesis | 23 |
| TLR4 signaling promotes B-cell maturation | 43 | |
| Toll-like receptor activation in human monocytes induces rapid differentiation DC-SIGN+CD16+ macrophages and CD1b+DC-SIGN− dendritic cells | 44 | |
| TLR7 stimulation at epithelial surfaces drives emergency myelopoiesis | 29 | |
| TLR2 activation results in HSC expansion in both the BM and spleen, increased HSC cycling, and a reduction in BM megakaryocyte-erythroid progenitors | 31 | |
| RIG-I MAVS signaling | Controls the emergence of hematopoietic precursors in zebrafish embryos through downstream IFN signaling | 33 |
| Activation of RIG-1 by repetitive element RNA, expressed during the endothelial-to-hematopoietic transition modulates the inflammatory signaling ie, crucial for HSPC formation during development | 34 | |
| cGAS-STING signaling | cia-cGAS is a suppressor of cGAS. deficiency of cia-cGAS promotes STING activation leading to increased HSC cycling and cGAS-mediated HSC exhaustion | 45 |
| Excessive R-loop accumulation because of DDX41 insufficiency, results in cGAS-STING-NF-κB pathway activation to promote HSPC expansion | 35 | |
| Activation of STING pathway by bacterial c-di-GMP activates in a cGAS-independent manner, induces HSPC proliferation, and mobilization as well as myeloid-biased differentiation, but reduces HSC self-renewal and mobilization | 46 |
| Microbiome-driven signaling pathway . | Effects on hematopoietic system . | Reference . |
|---|---|---|
| NOD1 signaling | Promotes steady-state hematopoiesis indirectly through the induction of cytokines by mesenchymal stromal cells | 42 |
| RAC1-NOD1-RIPK2-NF-κB axis initiates inflammatory signaling in the HE, resulting in HSPC specification | 25 | |
| NOD1 signaling in hematopoietic compartment regulates proinflammatory macrophage polarization and neutrophil infiltration in adipose tissue | 22 | |
| TLR-MYD88 signaling | Bacterial recognition during infection promotes the G-CSF secretion from endothelial cells and monocytes, leading to the acceleration of granulopoiesis | 23 |
| TLR4 signaling promotes B-cell maturation | 43 | |
| Toll-like receptor activation in human monocytes induces rapid differentiation DC-SIGN+CD16+ macrophages and CD1b+DC-SIGN− dendritic cells | 44 | |
| TLR7 stimulation at epithelial surfaces drives emergency myelopoiesis | 29 | |
| TLR2 activation results in HSC expansion in both the BM and spleen, increased HSC cycling, and a reduction in BM megakaryocyte-erythroid progenitors | 31 | |
| RIG-I MAVS signaling | Controls the emergence of hematopoietic precursors in zebrafish embryos through downstream IFN signaling | 33 |
| Activation of RIG-1 by repetitive element RNA, expressed during the endothelial-to-hematopoietic transition modulates the inflammatory signaling ie, crucial for HSPC formation during development | 34 | |
| cGAS-STING signaling | cia-cGAS is a suppressor of cGAS. deficiency of cia-cGAS promotes STING activation leading to increased HSC cycling and cGAS-mediated HSC exhaustion | 45 |
| Excessive R-loop accumulation because of DDX41 insufficiency, results in cGAS-STING-NF-κB pathway activation to promote HSPC expansion | 35 | |
| Activation of STING pathway by bacterial c-di-GMP activates in a cGAS-independent manner, induces HSPC proliferation, and mobilization as well as myeloid-biased differentiation, but reduces HSC self-renewal and mobilization | 46 |
cGAS, cyclic GMP-AMP synthase; GMP, guanosine monophosphate; HE, hemogenic endothelium.
Microbiome regulation of myeloid cells
Signals from the microbiota mediate myeloid cell homeostasis and function in multiple tissues. Signals from the microbiome are critical for the induction of hematopoietic growth factors and cytokines such as G-CSF, GM-CSF, SCF, IL-7, IL-3, IL-7, Flt3L, and TPO that collectively support HSPC and myeloid cell maintenance.22,47-50 Recently, Kovtonyuk et al demonstrated that aging-associated leaky gut is associated with increased translocation of microbial compounds, elevated IL-1a/b, and myeloid differentiation of HSCs. Antibiotic treatment reduced the myeloid bias and restored a balanced lineage output by suppressing IL-1 production, highlighting a critical role of the microbiome in driving HSC inflammaging.51 Deshmukh et al reported that exposing pregnant mouse dams to antibiotics disrupted microbiome composition in the neonatal gut and suppressed neutrophils and GMPs in the BM of neonates.47 As noted above, GF mice have reduced granulocytes and monocytes at baseline, and counts can be restored upon oral administration of NOD1L, a bacterial cell wall component.22,50 Interestingly, it has been shown that activation of the NOD1 signaling pathway by intestinal bacteria governs the survival and turnover of neutrophils and monocytes in an IL-17A-dependent manner.50 Similarly, Chen et al used a GF fate-mapping transgenic mouse model to show that the microbiota dictates steady-state turnover and lifespan of F4/80hi macrophages in the lamina propria by modulating the expression of cytokines and chemokines in the intestinal micro environment.52 Antiapoptotic effects on neutrophils have also been reported upon stimulation with polyinosinic:polycytidylic acid and lipopolysaccharide.53 Additionally, induction of serum amyloid A by commensal microbiota activates the NF-KB pathway to promote neutrophil migration in zebrafish. Consistent with gnotobiotic mammals, conventionalized zebrafish had increased numbers of neutrophils and other myeloid markers, suggesting an evolutionarily conserved role for enteric commensals in controlling production and turnover of myeloid cells.52
Neutrophil function has also been found to be influenced by the microbiome. Clarke et al showed that peptidoglycan from commensal Escherichia coli translocated from gut to the BM where it primed and enhanced the pathogen clearing function of neutrophils.54 Toll-like receptor adapter proteins such as MyD88, TIRAP, TRIF, or TRAM can regulate specific aspects of cell state and function when activated by molecular cues from the microbiome.55 Zhang et al found that activation of Myd88 mediates neutrophil aging under steady state,56 whereas TRIF is important for homeostatic, G-CSF–dependent feedback regulation of neutrophil granulopoiesis.57 Adult mice treated with antibiotics showed markedly reduced aged neutrophil counts and NET formation, whereas gavage of TLR4 ligand lipopolysaccharide completely restored aged neutrophils.56
Several studies have recently reported that signals derived from the microbiome govern various aspects of basophil and eosinophil homeostasis. Hill et al reported that commensal-derived signals control the production and development of basophil populations in a IgE–dependent manner. Furthermore, signals from the microbiome limit the proliferative capacity of BM-resident basophil precursor populations by modulating surface-bound CD123 and responsiveness to IL-3.42 In comparison to standard specific pathogen free mice, GF mice have decreased eosinophil count;58 these eosinophils have smaller granules with reduced cytoplasmic content. Furthermore, profound defects in IL-11 and CXCL9 in GF mice impair eosinophil migration and infiltration resulting in relative eosinophilia in GF conditions.59
Previous studies have shown that dendritic cells sense commensal-derived molecules through Syk-kinase-coupled C-type lectin receptors to induce cytokine, IgA, and antimicrobial peptide production to promote gut barrier integrity.60 Schaupp et al reported that redundant signaling through pattern recognition receptors using adapter proteins such as MyD88, TRIF, and/or CARDIF (also known as mitochondrial antiviral signaling protein [MAVS]) is required for constitutive IFN-I production by pDCs.61 This tonic IFN-I signaling instructs the epigenetic and metabolic basal state of conventional DCs, thereby priming them to effectively respond to microbial encounters. Similarly, signaling through Myd88 was found to be important for inducing the IgA class-switching function of lung dendritic cells in a TGF-β-dependent manner.62 Bacterial byproducts may also indirectly regulate DCs via other cell types. Short chain fatty acids (SCFAs) produced by commensal bacteria have been shown to activate retinaldehyde dehydrogenase 1 expression in intestinal epithelial cells resulting in retinoic acid production, enhanced intestinal barrier function, and tolerogenic CD103+ DC phenotypes in the gut.63,64 Finally, although the microbiota is dispensable for the development, differentiation, or maturation of splenic natural killer (NK) cells, it is required to prime NK effector functions. Splenic NK cells in antibiotic-treated and GF mice exhibited impaired IFN-γ production and cytotoxicity.65
Microbiome regulation of lymphoid cells
The microbiota plays an essential role in the differentiation and function of T cells, including helper T (Th) 1, Th2, Th17, and regulatory T (Treg) cells. Mazmanian et al reported that bacterial polysaccharide from commensal Bacteroides fragilis drives systemic CD4+ T-cell maturation and development of lymphoid-organs and also directs DCs to signal TH1/TH2 lineage differentiation via regulation of Stat4.66 Conversely, cell surface β-glucan/galactan polysaccharides and outer membrane vesicles of Bifidobacterium bifidum serve as potent inducers of peripherally induced Treg cells.67 Tregs are also known to be induced by methylated CpG motif derived from Bifidobacterium longum subsp. infantis.68 Indeed, consumption of B. infantis 35624 enhanced generation and activity of Treg cells that protected mice during Salmonella typhimurium infection by reducing CD4 T-cell proliferation and diminishing NF-κB activation via TLR4 pathway.69 Similarly, intestinal colonization with Clostridium resulted in the induction of both proinflammatory and regulatory T cells via production of IFN-γ, IL-17, IL-13, IL-4, CD40L, and IL-10, all of which are known to orchestrate T-cell differentiation.70,71Prevotella colonization has also been shown to be associated with induction of Th17 related cytokines, IL-17A, and IL-6 in mouse serum and subsequent accumulation of colonic Th17 cells.72 Zeng et al recently reported that fecal microbiota transplant (FMT) from young mice to aged mice not only enhanced the absolute number and repopulation capacity of ST-HSCs and LT-HSCs but also restored lymphoid differentiation in aged LT-HSCs. Transplanted aged mice showed significantly lower expression of inflammatory markers (IFN-γ, IL-6, and IL-4), improved intestinal barrier integrity, and increased activation of forkhead box O1 pathway, which has been shown to protect HSC from DNA damage and mitigate HSC aging.73
The gut microbiota influences B-cell differentiation and activation via direct and indirect mechanisms. The depletion of the gut microbiota is associated with reduction in regulatory B-cell numbers in the spleen and IL-10 and IL-35 in circulation. Administration of bacterial DNA of the microbiota can rescue these defects thereby preventing initiation of lupus in antibiotic-treated mice.74 Conversely, treatment with antibiotics negatively affects the number and function of regulatory B cells in the spleen and mesenteric lymph nodes.75 Finally, a recent study found that butyrate, a short chain fatty acid produced by the intestinal microbiota, activates the aryl-hydrocarbon receptor to support regulator B-cell generation and function, while concomitantly inhibiting germinal center B cell and plasmablast differentiation.76
Microbial signaling strongly influences B-cell function. Combinatorial signaling through TLR2 and CD86, which can be activated by a variety of microbial products such as neisserial porins and pneumococcal surface adhesin A, drives the maturation of resting B cells via TLR2.CD86 to reduce expression of proapoptotic genes and improve survival with elevated levels of IgG1 and IgM and improved antigen uptake capacity.77 Likewise, γ-D-glutamyl-meso-diaminopimelic acid found in cell wall of gram-negative commensal bacteria has been shown to activate Nod1 signaling during B-cell development to preferentially promote B-cell survival and persistence while also rescuing the cells from B-cell receptor–induced tolerance.78 Finally, commensal-derived short chain fatty acids (SCFAs) has been shown to directly influence the glycolytic activity of B cells in part through the activation of AMP-activated protein kinase and mTOR pathways, resulting in plasma cell differentiation and elevated Ig class-switch recombination activity.79
Altogether, there is ample evidence that the microbiota signals to the hematopoietic system to affect not only cell production at the progenitor level but also effector cell persistence and function, as summarized in Table 2. Therefore, it makes sense that the microbiome may affect hematopoietic disease states and treatment outcomes, as we discuss below.
Role of the microbiome-derived molecules in promoting immune cell homeostasis
| Microbial products . | Effects on hematopoietic system . | Reference . |
|---|---|---|
| Commensal-bacterial DNA motif CpG | Regulator of steady-state serum IgE levels and levels of circulating basophil populations | 42 |
| Promote the differentiation of GMPs and MDPs | 80 | |
| SCFAs | Lactate promotes SCF secretion by LepR+ BM MSCs, which activates hematopoiesis and erythropoiesis in a Gpr81-dependent manner | 24 |
| Restores colonic Treg numbers in GF mice | 81 | |
| Butyrate increases Ly6C− patrolling monocytes and interstitial macrophages in the lung tissue and promotes the differentiation of BM cells into macrophages in vitro | 82 | |
| Propionate administration increases DC precursors and modulates DC function | 83 | |
| Microbiota-derived butyrate promotes emergency erythrophagocytosis by BM macrophages to distribute iron that controls HSC self-renewal and differentiation | 84 | |
| Maternally derived SCFAs have been suggested to play a role in Foxp3+ regulatory T-cell generation in the neonatal thymus | 85 | |
| Lipopolysaccharide | Drives myeloid differentiation in a MyD88-dependent manner in vitro | 28 |
| Increases the proliferation and self-renewal capacity of HSC | 86 | |
| Regulates neutrophil activation and aging under steady-state conditions | 56 | |
| Peptidoglycan (NODL1) | Rescue antibiotic–induced granulocytic defects | 87 |
| Primes neutrophils for optimal oxidative and nonoxidative bacterial killing mechanisms | 54 | |
| Restores the number of HSCs, MPPs, CMPs, and granulocytes in the BM of GF and antibiotic-treated mice | 22 | |
| Regulates steady-state cellular lifespan and turnover of neutrophils and inflammatory monocytes | 50 | |
| poly(I:C) | Prolongs PMN survival and enhanced respiratory burst ability | 53 |
| MAMPs (heat killed E coli) | Sufficient to rescue GMP-mediated expansion of neutrophils and monocytes in GF mice | 88 |
| Microbial products . | Effects on hematopoietic system . | Reference . |
|---|---|---|
| Commensal-bacterial DNA motif CpG | Regulator of steady-state serum IgE levels and levels of circulating basophil populations | 42 |
| Promote the differentiation of GMPs and MDPs | 80 | |
| SCFAs | Lactate promotes SCF secretion by LepR+ BM MSCs, which activates hematopoiesis and erythropoiesis in a Gpr81-dependent manner | 24 |
| Restores colonic Treg numbers in GF mice | 81 | |
| Butyrate increases Ly6C− patrolling monocytes and interstitial macrophages in the lung tissue and promotes the differentiation of BM cells into macrophages in vitro | 82 | |
| Propionate administration increases DC precursors and modulates DC function | 83 | |
| Microbiota-derived butyrate promotes emergency erythrophagocytosis by BM macrophages to distribute iron that controls HSC self-renewal and differentiation | 84 | |
| Maternally derived SCFAs have been suggested to play a role in Foxp3+ regulatory T-cell generation in the neonatal thymus | 85 | |
| Lipopolysaccharide | Drives myeloid differentiation in a MyD88-dependent manner in vitro | 28 |
| Increases the proliferation and self-renewal capacity of HSC | 86 | |
| Regulates neutrophil activation and aging under steady-state conditions | 56 | |
| Peptidoglycan (NODL1) | Rescue antibiotic–induced granulocytic defects | 87 |
| Primes neutrophils for optimal oxidative and nonoxidative bacterial killing mechanisms | 54 | |
| Restores the number of HSCs, MPPs, CMPs, and granulocytes in the BM of GF and antibiotic-treated mice | 22 | |
| Regulates steady-state cellular lifespan and turnover of neutrophils and inflammatory monocytes | 50 | |
| poly(I:C) | Prolongs PMN survival and enhanced respiratory burst ability | 53 |
| MAMPs (heat killed E coli) | Sufficient to rescue GMP-mediated expansion of neutrophils and monocytes in GF mice | 88 |
CMPs, common myeloid progenitors; IgE, immunoglobulin E; MDP, monocyte and dendritic cell progenitor; MPPs, multipotent progenitors; PMN, polymorphonuclear leukocyte.
Microbiome regulation of premalignant hematopoiesis
Research on somatic mutations that drive clonal hematopoiesis and their risk for transformation into hematologic malignancies has exponentially bloomed over the last few decades.39,89 Although not the primary focus of this review, several studies demonstrate the influence of the microbiome on evolution of clonal hematopoiesis into preleukemic states. Meisel et al showed that preleukemic myeloproliferation was induced by disruption of intestinal barrier, bacterial translocation, and increased IL-6 production in mice lacking Tet2.90 Similarly, Caiado et al demonstrated that increased microbial-dependent inflammatory cytokines seen with aging leads to increased IL-1 signaling, which in turns drives Tet2 clonal expansion in mouse models of Tet2+/−-driven clonal hematopoiesis of indeterminate potential.91 Further, research may allow for identification of microbiome-related drivers of clonal hematopoiesis and may serve as therapeutic targets that prevent malignant evolution.
Effect of microbiome in HSC transplant, immunotherapy, and cellular therapy outcomes
Hematopoietic stem cell transplantation (HSCT) has long been used as a curative treatment for treatment resistant hematologic malignancies and immune deficiencies. More recently, immunotherapy, with immune checkpoint inhibitors, adoptive T-cell therapy including chimeric antigenic receptor (CAR) T-cell therapy, and others are being used increasingly and successfully to treat a wide array of hematologic and solid tumor malignancies. Over the last decade, a rapidly growing body of evidence illustrates how the intestinal microbiota correlates with cancer-directed therapies, their antitumor effects and toxicities as well as outcomes for patients in terms of overall survival (OS) and disease free-survival. Here, we discuss those interactions as well as specific bacterial taxa noted to influence outcomes of these cancer-directed therapies (Table 3).
Changes in abundance of specific bacterial taxa are associated with changes in outcomes of HSCT and cellular therapies
| Bacterial taxa . | Study findings . | Reference . |
|---|---|---|
| Associations with positive outcomes | ||
| Blautia | Decreased need for aGVHD systemic therapy, decreased GHVD-related and relapse-related mortality, and improved OS | 92 |
| Lachnospiraceae | Reduced incidence of lethal GVHD | 93 |
| Lachnospiraceae, Actinomycetaceae | Greater abundance in patients who survived after HSCT | 93 |
| Faecalibacterium, Ruminococcus, Akkermansia | Increased neutrophil engraftment | 94 |
| Ruminococcus, Staphylococcus | Increased lymphocyte engraftment | 95 |
| Clostridium spp | Loss of Clostridium is associated with worse survival and increased GVHD rates | 95 |
| Ruminococcaceae, Oscillospiraceae | Higher abundance in higher diversity group noted to have improved OS and lower incidence of grade 2-4 aGVHD | 96 |
| Eubacerium limosum | Higher abundance correlated with decreased risk of relapse/disease progression | 97 |
| Bifidobacterium | Improved antitumor effect of CTLA-4 inhibitor in mice | 98 |
| Firmicutes | Improved antitumor effect of CTLA-4 inhibitor in humans | 99 |
| Lachnospiraceae, Ruminococcaceae | Prevalent in patients who achieved complete remission after CAR T-cell therapy | 100 |
| Bacteria from Clostridia class, including the genera Ruminococcus and Faecalibacterium, the family Ruminococcaceae, and the species Faecalibacterium prausnitzii and Ruminococcus bromii | Higher abundance correlated with day 100 complete response and lack of toxicity after CD-19 CAR T-cell | 101 |
| Bacteroides, Ruminococcus, Eubacterium, and Akkemansia | Correlated with CD-19 CAR T-cell response in patients not exposed to high-risk antibiotic therapy | 102 |
| Associations with negative outcomes | ||
| Veillonella | Increased GVHD–related mortality | 92 |
| Gammaproteobacteria (Enterobacteriaceae) | Greater abundance in patients who died after HSCT | 93 |
| Rothia, Clostridium sensu stricto 1 | Reduced neutrophil engraftment | 94 |
| Enterococcus (including E faecalis and E faecium) | Domination in the early post-SCT period is associated with decreased OS and increased GVHD–related mortality in humans and mice | 95 |
| Enterococcaceae and Enterobacteriaceae | Higher abundance in lower diversity group noted to have worse OS and higher incidence of grade 2-4 aGVHD | 96 |
| Peptostreptococcaceae and Clostridiales | Prevalent in nonresponders after CAR T-cell therapy | 100 |
| Veillonellaceae | Decreased d 100 complete response after CD-19 CAR T-cell therapy | 101 |
| Staphylococcus | Decreased CD4 T-cell recovery | 103 |
| Bacterial taxa . | Study findings . | Reference . |
|---|---|---|
| Associations with positive outcomes | ||
| Blautia | Decreased need for aGVHD systemic therapy, decreased GHVD-related and relapse-related mortality, and improved OS | 92 |
| Lachnospiraceae | Reduced incidence of lethal GVHD | 93 |
| Lachnospiraceae, Actinomycetaceae | Greater abundance in patients who survived after HSCT | 93 |
| Faecalibacterium, Ruminococcus, Akkermansia | Increased neutrophil engraftment | 94 |
| Ruminococcus, Staphylococcus | Increased lymphocyte engraftment | 95 |
| Clostridium spp | Loss of Clostridium is associated with worse survival and increased GVHD rates | 95 |
| Ruminococcaceae, Oscillospiraceae | Higher abundance in higher diversity group noted to have improved OS and lower incidence of grade 2-4 aGVHD | 96 |
| Eubacerium limosum | Higher abundance correlated with decreased risk of relapse/disease progression | 97 |
| Bifidobacterium | Improved antitumor effect of CTLA-4 inhibitor in mice | 98 |
| Firmicutes | Improved antitumor effect of CTLA-4 inhibitor in humans | 99 |
| Lachnospiraceae, Ruminococcaceae | Prevalent in patients who achieved complete remission after CAR T-cell therapy | 100 |
| Bacteria from Clostridia class, including the genera Ruminococcus and Faecalibacterium, the family Ruminococcaceae, and the species Faecalibacterium prausnitzii and Ruminococcus bromii | Higher abundance correlated with day 100 complete response and lack of toxicity after CD-19 CAR T-cell | 101 |
| Bacteroides, Ruminococcus, Eubacterium, and Akkemansia | Correlated with CD-19 CAR T-cell response in patients not exposed to high-risk antibiotic therapy | 102 |
| Associations with negative outcomes | ||
| Veillonella | Increased GVHD–related mortality | 92 |
| Gammaproteobacteria (Enterobacteriaceae) | Greater abundance in patients who died after HSCT | 93 |
| Rothia, Clostridium sensu stricto 1 | Reduced neutrophil engraftment | 94 |
| Enterococcus (including E faecalis and E faecium) | Domination in the early post-SCT period is associated with decreased OS and increased GVHD–related mortality in humans and mice | 95 |
| Enterococcaceae and Enterobacteriaceae | Higher abundance in lower diversity group noted to have worse OS and higher incidence of grade 2-4 aGVHD | 96 |
| Peptostreptococcaceae and Clostridiales | Prevalent in nonresponders after CAR T-cell therapy | 100 |
| Veillonellaceae | Decreased d 100 complete response after CD-19 CAR T-cell therapy | 101 |
| Staphylococcus | Decreased CD4 T-cell recovery | 103 |
CTLA-4, cytotoxic T-lymphocyte associate protein 4.
The intestinal microbiota and HSCT
Since the 1970s, the impact of intestinal commensals in HSCT outcomes was proposed after it was noted that mice kept under GF conditions or treated with gut-decontaminating antibiotics developed less gut graft-versus-host disease (GVHD).104,105 A more nuanced understanding of this relationship has evolved, as it is now recognized that whereas pathogen associated molecular patterns may provide immunostimulation to trigger GVHD, commensal metabolites are critical to maintain intestinal lumen integrity.94 Indeed a recent report showed GF mice have greater GVHD, and microbiome repletion and diversity are crucial for lessening GVHD severity.106 Recent preclinical and clinical studies have further described the influence of the gut microbiota on HSCT clinical outcomes such as OS, transplant-related mortality (TRM), incidence, and severity of GVHD, immune reconstitution, relapse rate, and others.107
Transplant Outcomes: OS, TRM, and GVHD
Low bacterial diversity has been associated with poor outcomes after HSCT. Intense conditioning regimens with chemotherapeutic agents and total-body irradiation, together with the use of prophylactic broad-spectrum antibiotics for patients who have undergone HSCT allow for selection and overgrowth of pathogenic intestinal species and compromise the intestinal barrier, in turn facilitating bacterial translocation and infection. Taur et al93 analyzed peri-engraftment fecal specimens from 80 allogeneic-HSCT (allo-HSCT) recipients and reported worse OS and TRM, GVHD–related and infection-related mortality for subjects with low microbial diversity, even after adjusting for pretransplant comorbidity, disease risk, and antibiotic administration. Microbiotas from subjects with low diversity were generally dominated by a single bacterial genus including Enterococcus, Streptococcus, Enterobacteriaceae, or Lactobacillus. Colonization by antibiotic-resistance bacteria is of particular concern as it has been linked to poorer OS, higher incidence of grade 2-4 acute GVHD (aGVHD) and infection-related mortality.87,108 Similarly, a recent study demonstrated a correlation between low gut microbial diversity, worse OS, and higher incidence of grade 2-4 aGVHD in a cohort of 90 pediatric allogeneic stem cell transplantation (allo-SCT) recipients. This study also showed higher abundances of Enterococcaceae and Enterobacteriaceae in the low diversity group, whereas the high diversity group had an increased representation of Ruminococcaceae and Oscillospiraceae.96
Loss of intestinal diversity and single bacterial taxa domination have been implicated in the pathogenesis of GVHD; conversely, specific taxa have been noted to have a protective effect against it.92,93 In a prospective study of 115 allo-SCT recipient patients, stool composition analysis showed an association between increased bacterial diversity and abundance of bacteria from the genus Blautia on day 12 after HSCT and reduced acute GVHD (aGVHD)–related mortality with improved OS. In this study, loss of Blautia was associated with longer courses of total parenteral nutrition and treatment with anaerobe-active antibiotics.92 Similarly, a retrospective report found that early antibiotic treatment pre-HSCT correlated with lower urinary levels of 3-indoxyl sulfate (3-IS) (an indirect marker for Clostridium species), decreased OS, and increased TRM and GVHD–related mortality.99 Thus, Blautia and Clostridia may both be protective against GVHD.
Conversely, several species have been associated with increased risk of GVHD. Veillonella increased GVHD–related mortality,92 as does fecal domination by Enterococcus in both a large cohort of adult allo-HSCT recipients and mice that developed lethal aGVHD after SCT.95 Enterococcal expansion after allogeneic hematopoietic cell transplantation (allo-HCT) was accompanied by a loss of Clostridium spp. in the microbiota of patients who have undergone allo-HCT and of mice with GVHD. Importantly, microbiome analysis in one of the largest cohorts of allo-HSCT recipients reported to date again demonstrated loss of diversity, single taxa domination, and higher TRM and GVHD–related mortality; with low diversity characterized by abundance of Enterococcus, Klebsiella, Escherichia, Staphylococcus, and Streptococcus.109
Aside from bacterial species, specific microbiome-derived metabolites have also been implicated in GVHD mitigation. Mathewson et al reported decreased butyrate concentration in the intestinal tissues of mice 7 days after SCT, resulting in decreased histone acetylation.110 Exogenous butyrate administration restored histone acetylation and intestinal epithelial cell junctions; and intragastric gavage of butyrate-producing Clostridia strains pre- and peritransplant decreased GVHD severity compared with control mice. Another study linked the domination of Enterococcus spp. and loss of Clostridium spp. with a reduction in fecal butyrate and enrichment of pathways involved in DNA synthesis and, notably, in lactose and galactose degradation.95
Relapse or immune reconstitution
Characterization of risk factors for relapsed disease after HSCT is an active area of research; however, studies linking risk of relapse to microbiome changes are sparse. A prospective study noted higher abundance of Eubacterium limosum to be associated with decreased risk of relapse or disease progression in a cohort of 541 undergoing allo-HSCT.97 Schluter et al compared peripheral blood counts between allo-HSCT recipients who did and who did not receive FMT during a clinical trial. Higher white blood cell counts were observed in individuals who received an auto-FMT during the first 100 days after neutrophil engraftment.94 Additionally, fecal microbial diversity was found to be an independent predictor of CD4 T-cell count after allo-HSCT in a cohort of 894 patients.103
Therapeutic approaches and microbiome manipulation in the setting of HSCT
Understanding the interactions between the gut microbiota, host immunity, and SCT could pave the way for modifications of clinical management with the goal of preserving the microbiome, preventing HSCT morbidities, and improving survival. To this end, dietary changes in conjunction with prebiotic administration has been proposed to aid in preserving the microbiota before and after SCT. Supplementation of diet with glutamine, fiber, and a fructo-oligosaccharide has been noted to decrease days of diarrhea and mucositis after transplant.111 Similarly, supplementation with sucrose in the drinking water improved post-BM transplant hematopoietic recovery in mice with depleted intestinal flora.112 Other attractive therapeutic targets include SCFA and small molecules that aid in SCFA production via interaction with G-protein–coupled receptors or fermentative enzymes. Finally, a lactose-free diet has also been proposed as a therapeutic strategy in light of the lactose-dependent nature of certain pathogenic bacteria, but efficacy of this approach has not been reported.95
The administration of probiotics has been a focus of active research and clinical trials, particularly to address safety concerns about probiotic administration in severely immunocompromised patients. Pilot studies have demonstrated the safety and feasibility of Lactobacillus plantarum as a probiotic in pediatric patients undergoing HSCT.113 Additionally, significant preclinical work is being done to develop precision prebiotics and symbiotics that favor the growth and restoration of commensal SCFA producers and prevent the overgrowth of pathogenic species.114,115 Meanwhile, antibiotic strategies designed to preserve microbiome diversity while treating infections adequately are under investigation, including regimens such as clostridia-sparing β-lactams that protect species related to improved SCT outcomes.
FMT is another strategy to achieve a predefined composition that may be protective against pathogenic organism blooms, GVHD, or other major complications. Spindelboeck et al reported safe use of FMT in 3 patients with severe refractory gut aGVHD. Although microbiome engraftment was short-lived and required repeated FMT, the patients experienced sustained symptom and endoscopic improvement.116 Importantly, no infections immediately related to FMT were noted in this cohort. In another study, use of a pooled allogeneic microbiome ecosystem therapy MaaT013 in patients with steroid refractory GVHD showed a 38% response rate and was associated with higher microbiome diversity.117 FMT in the setting of SCT is actively being studied in dozens of clinical trials worldwide, and guidelines on optimal donor selection, auto FMT vs FMT from healthy donor preferences are still in development.
Immune checkpoint inhibitors
Immune checkpoint inhibitors (ICI) have revolutionized the treatment of solid and hematologic malignancies over the last several decades; however, factors affecting toxicities and treatment response have not been entirely elucidated. Emerging data on how the microbiome affects the efficacy of ICI has recently been reported. Bifidobacterium administration in a melanoma-murine model improved tumor control by CTLA-4 inhibitor ipilimumab,98 and in human patients with metastatic melanoma, anticancer response to ipilimumab correlated with Firmicutes colonization.118 With the advent of PDL-1 blockers for hematologic malignancies such as non-Hodgkin lymphoma, multiple myeloma, and leukemia; further research to understand how the microbiome influences antitumor effects is needed.
Cellular therapy
The use of CAR T cells dramatically altered the landscape of hematologic malignancies treatment, especially for relapsed-refractory disease. Its success continues to be limited by the long-term loss of antitumor efficacy and toxicities.119 Recent studies show that antibiotics and gut microbes affect the efficacy and toxicity of CAR T cells. One study showed that broad-spectrum antibiotic administration in mice was associated with prolonged persistence of CD-19 CAR T cells and increased duration of treatment effect.120 A retrospective study examining antibiotic exposure before CAR T-cell therapy found that exposure to piperacillin/tazobactam, meropenem, and imipenem/cilastatin in the 4 weeks before therapy was associated with worse survival and increased neurotoxicity.101 In this study, species within the Clostridia family were positively associated with day 100 complete response. A recent meeting abstract reported that Lachnospiraceae and Ruminococcaceae were found in patients achieving complete remission, whereas Peptostreptococcaceae and Clostridiales were more abundant in nonresponders.100 Conversely, Uribe-Herranz et al reported enhanced antitumor effects of CD-19 CAR T-cell therapy in mice and humans after vancomycin administration.121 Additionally, patients with B-cell acute lymphoblastic leukemia exposed to oral vancomycin showed higher CAR T-19 peak expansion compared with unexposed patients. Interestingly, another study found significant correlations between baseline Bifidobacterium longum abundance and 6-month survival after CD-19 CAR T-cell therapy.102 These authors developed and validated a predictive algorithm that separates responders from nonresponders based on Bacteroides, Ruminococcus, Eubacterium, and Akkermansia abundance. Altogether there is significant evidence that microbiome composition can affect CAR T-cell outcomes, but the details and mechanisms remain obscure.
Knowledge gaps, ongoing challenges, and future directions
The role of the intestinal microbiome in hematopoiesis, carcinogenesis, cancer therapy, and SCT outcomes is burgeoning. Although studies have begun to uncover the mechanisms by which the microbiome communicates with the distant BM to fine-tune hematopoiesis both at steady-state and during physiological stress, there are many details that remain unknown. Some of the most important unanswered questions that remained are as follows: which bacterial species contribute to normal blood production? Which cell types receive the signals from the microbiota to sustain steady-state hematopoiesis? What commensally derived microbial metabolites can promote myelopoiesis? Understanding these questions will help support strategies to prevent cytopenia in patients who require prolonged antibiotics, patients recovering from cytotoxic chemotherapy, or patients who have received SCT.
We currently understand microbial diversity as a determinant of SCT outcomes and GVHD prevention and severity; however, further research is needed to help establish mechanistic and molecular pathways that prove causality between the intestinal microbiome and response to hematologic malignancies-directed therapies such as SCT, ICI, and cellular therapies. In this arena, the role of the microbiome in relapsed disease after SCT and CAR T-cell therapy nonresponse remains obscure. This research is directed at defining the role of FMT in SCT and other therapies, and identifying biomarkers that may help predict response to cellular therapy and SCT outcomes. It will be fascinating to discover whether precision prebiotics and pooled microbiome products for FMT can be effective in reliably shifting microbiome diversity and improving outcomes; and it is interesting to speculate whether changes in the microbiome influence development of clonal hematopoiesis and malignancy risk.122
Clinical assessment of microbiome function through metagenomic sequencing or metabolomics may soon become clinically actionable. For example, high urinary concentrations of 3-indoxyl sulfate could identify patients with low microbiome diversity who might benefit from additional GVHD prophylaxis or interventions aimed at preserving the microbiome. Alternatively, expression of antimicrobial peptides in the gastrointestinal tract, such as human defensins 5 and 6 and regenerating islet-derived 3α, might be used as a surrogate marker for severe aGVHD risk according to findings reported by Weber et al.123,124 Developing modern “designer microbiota modulators” may be of use even in highly immunocompromised patients undergoing HSCT. Novel approaches that protect or restore the microbiota need to be developed in preclinical studies and further validated in clinical trials before the standard of care for HSCT, ICI, and cellular therapies recipients can be systemically changed.
Acknowledgments
The authors thank Kritika Bisht for helping with the figure. The authors thank their collaborators Robert Jenq, Robert Britton, Pavan Reddy, Ritu Banerjee, and Megan Baldridge and her laboratory members Forrest Walker and Lila Nolan for excellent discussions over the years. Former and present members of the King laboratory, including Kamilla Josefsdottir, Hannah Yan, and Hyojeong Han, contributed significantly to this review. The figure was created with BioRender.com.
This work was supported by grants from the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (R01AI141716), NIH, National Heart, Lung, and Blood Institute (NHLBI) (R35HL155672), NIH, National Cancer Institute (P01CA265748) (K.Y.K.), and NIH/NHLBI (F31HL168921) and NIH, National Institute of General Medical Sciences (T32GM136554) (A.A.M.). J.F.S. was supported by Baylor College of Medicine Comprehensive Cancer Training Program via Cancer Prevention & Research Institute of Texas Training Award #RP210027.
Authorship
Contribution: J.F.S. and A.A.M. developed the table and wrote the first draft of the manuscript; and K.Y.K. edited the draft, drew the graphical abstract, and provided feedback on all aspects.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katherine Y. King, Department of Pediatrics, Baylor College of Medicine, 1102 Bates St, Suite 1150, Houston, TX 77030; email: kyk@bcm.edu.
References
Author notes
J.F.S and A.A.M contributed equally to this study.
Data are available upon reasonable request from the corresponding author, Katherine Y. King (kyk@bcm.edu).


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal