Neoantigens arising from recurrent AML mutations are immunogenic and represent novel immunotherapeutic targets.
IDH2R140Q-specific T cells can selectively eliminate neoantigen/HLA-matched AML in vitro and in vivo.
Visual Abstract
For patients with high-risk or relapsed/refractory acute myeloid leukemia (AML), allogeneic stem cell transplantation (allo-HSCT) and the graft-versus-leukemia effect mediated by donor T cells, offer the best chance of long-term remission. However, the concurrent transfer of alloreactive T cells can lead to graft-versus-host disease that is associated with transplant-related morbidity and mortality. Furthermore, ∼60% of patients will ultimately relapse after allo-HSCT, thus, underscoring the need for novel therapeutic strategies that are safe and effective. In this study, we explored the feasibility of immunotherapeutically targeting neoantigens, which arise from recurrent nonsynonymous mutations in AML and thus represent attractive targets because they are exclusively present on the tumor. Focusing on 14 recurrent driver mutations across 8 genes found in AML, we investigated their immunogenicity in 23 individuals with diverse HLA profiles. We demonstrate the immunogenicity of AML neoantigens, with 17 of 23 (74%) reactive donors screened mounting a response. The most immunodominant neoantigens were IDH2R140Q (n = 11 of 17 responders), IDH1R132H (n = 7 of 17), and FLT3D835Y (n = 6 of 17). In-depth studies of IDH2R140Q-specific T cells revealed the presence of reactive CD4+ and CD8+ T cells capable of recognizing distinct mutant-specific epitopes restricted to different HLA alleles. These neo–T cells could selectively recognize and kill HLA-matched AML targets endogenously expressing IDH2R140Q both in vitro and in vivo. Overall, our findings support the clinical translation of neoantigen–specific T cells to treat relapsed/refractory AML.
Introduction
Acute myeloid leukemia (AML) is an aggressive hematological malignancy characterized by bone marrow infiltration with clonal myeloid precursor cells, resulting in life-threatening infections, hemorrhage, and anemia.1 At diagnosis, approximately one-quarter of patients have high-risk disease (based on genetic profiling) and require intensive chemotherapy and allogeneic hematopoietic stem cell transplantation (allo-HSCT) for the best chance of cure.2,3 These treatments are associated with significant complications, such as graft-versus-host disease (GVHD) and infections. Despite the aggressive intervention, ∼60% of patients relapse after allo-HSCT, resulting in a dismal 2-year overall survival rate of 10% to 25%.4-6 Thus, there is an urgent need for novel treatments that are specific, safe, tolerable, and associated with durable benefits.
Although adoptively transferred chimeric antigen receptor (CAR)-modified T cells have transformed the treatment of relapsed/refractory CD19+ hematological malignancies, successful extension to AML has been hindered by the lack of tumor-exclusive target antigens.7,8 For example, CD33 and CD123, expressed on the surface of leukemic blasts, are also present on normal hematopoietic stem cells, myeloid precursors, and granulocytes whose ablation is clinically intolerable.9 Fortunately, leukemic blasts express a rich pool of intracellular antigens that can be processed and presented in the context of major histocompatibility complex on the cell surface, making them potentially targetable by T cells via the native or transgenic T-cell receptors (TCR).10-13 Indeed, early-phase clinical trials of adoptively transferred T cells targeting leukemic-associated and cancer testes antigens (eg, WT1, survivin, PRAME, and NY-ESO-1) have demonstrated the benefit of this approach with evidence of safety as well as remitting blast counts and long-term remissions in some patients.13-15
Given this clinical promise, we sought to extend the spectrum of immunotherapeutic AML targets by exploring the immunogenicity of neoantigens, which arise from nonsynonymous mutations within malignant cells and thus represent targets exclusive to the tumor.16 We focused on 14 recurrent driver mutations, including DNMT3AR882C, R882H, IDH2R140Q, and NPM1Types A, B, and D.17-20 We now report on the immunodominance, phenotypic characteristics, and functional profile of T cells directed against our selected neoantigens, with specific focus on IDH2R140Q-reactive cells and their capacity to discriminate between tumor and normal cells, and to selectively mediate antitumor effects in vitro and in vivo.
Methods
Healthy donor samples
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy donors and patients with informed consent on Baylor College of Medicine institutional review board–approved protocols (H-45017 and H-43516). PBMCs from healthy donors were used to generate dendritic cells (DCs), neoantigen-specific T cells (neo-T cells), and OKT3-simulated T cells, as described hereafter. Primary AML blasts were isolated from patient PBMCs, as described hereafter.
Neo-T cells
Neoantigen peptides
To generate neo-T cells, we used the neoantigen mastermix (NeoMastermix), which comprised 14 individual neopools. Each neopool comprised 5 15mers overlapping by 11 amino acids (AAs) spanning each mutation, with the mutated AA at various positions (supplemental Figure 1; available on the Blood website). Minimal epitopes for CD8-mediated responses were mapped using 9mer and/or 10mer peptides overlapping by 8 or 9 AAs, respectively. Peptides were purchased from Genemed Synthesis (San Antonio, TX) and JPT Peptide Technologies (Berlin, Germany), and were reconstituted at 10 mg/mL in dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO).
Monocyte-derived DC generation
Monocytes were magnetically isolated from PBMCs using CD14 microbeads (Miltenyi Biotec, Cambridge, MA) according to the manufacturer’s instructions. These were cultured in DC medium (CellGenix USA, Portsmouth, NH) with granulocyte macrophage-colony stimulating factor (GM-CSF; 800 U/mL) and interleukin-4 (IL-4; 400 U/mL) for 5 days. Cytokines were replenished on day 2 or 3. On day 5 or 6, DCs were matured in DC medium supplemented with prostaglandin E1 (1 μg/mL; Sigma-Aldrich), IL-1β (10 ng/mL), tumor necrosis factor α (TNF-α; 10 ng/mL), IL-6 (100 ng/mL), GM-CSF (800 U/mL), and IL-4 (400 U/mL; all from R&D Systems).
In vitro expansion of neo-T cells
DCs loaded with the neoantigen mastermix, neopools, or individual peptides were cocultured with autologous PBMCs in T-cell medium (50% RPMI-1640 [Hyclone Laboratories, Logan, UT], 50% Clicks Medium [Irvine Scientific, Santa Ana, CA], 5% human AB serum [Valley Biomedical, Winchester, VA], and 1% GlutaMax [Gibco, Life Technologies, Gaithersburg, MD]) supplemented with a type 1 T helper cell (Th1)-polarizing cytokine cocktail (IL-7, 10 ng/mL; IL-12, 10 ng/mL; IL-15, 5 ng/mL [all from Peprotech, Rocky Hill, NJ]; and IL-6, 100 ng/mL). From day 10, responder T cells were restimulated weekly with peptide-pulsed DCs in the presence of IL-15, IL-2 (50-100 U/mL), and/or IL-7.
OKT3-activated T cells
OKT3-activated T cells (OKT3-blasts) were generated by activating PBMCs with anti-OKT3 (0.5 mg/mL; Ortho Biotech, Bridgewater, NJ) and anti-CD28 (0.5 mg/mL; BD Bioscience). Cells were maintained in T-cell medium with IL-2 (100 IU/mL), which was replenished every 2 or 3 days.
Tumor cell lines and primary AML blasts
The AML cell lines Kg1A, MOLM13, R140Q TF-1, and parental TF-1 were obtained from the American Type Culture Collection and maintained as per the manufacturer’s instructions. Cell line verification was performed using DNA short tandem repeat profiling (University of Arizona, Tucson, AZ). Primary AML blasts were selected from patient PBMCs using the CD34+ MicroBead Kit (Miltenyi) and maintained in StemSpan SFEM II supplemented with CD34+ Expansion Supplement (Stemcell Technologies, Vancouver, Canada), as per the manufacturer’s instructions. The modification of cell lines and primary AML blasts to express neoantigens and relevant HLA molecules is described in supplemental Methods.
Flow cytometry for immunophenotypic analysis
After 3 stimulations, neo-T cells were surface stained with the monoclonal antibodies for T/natural killer cell subsets (CD3 [BD Biosciences, Pasadena, CA], CD56 [BioLegend, San Diego CA], CD4 and CD8 [both Beckman Coulter, Brea, CA]), memory markers (CD45RO [BD Biosciences] and CD62L [Beckman Coulter]), activation markers (CD69 [BioLegend], CD28 and CD25 [both BD Biosciences]), and exhaustion markers (PD1 [BD Biosciences] and TIM3 [BioLegend]). Briefly, T cells were washed and pelleted, then antibodies were added in saturating amounts (2 μL). At least 50 000 live cells were acquired using a Gallios flow cytometer, and data were analyzed using Kaluza software (version 1.3, Beckman Coulter).
Functional assays
Enzyme-linked immunosorbent spot (ELISpot) and FluoroSpot
Unless otherwise stated, all functional analysis was performed on pan (nonselected) T cells. PBMCs (5 × 105) or neo-T cells (0.5 × 105 to 2 × 105) were seeded per well in 96-well ELISpot or FluoroSpot plates and exposed to individual peptides or pepmix, with phytohemagglutinin (1 mg/mL) and unstimulated cells serving as positive and negative controls, respectively. The plates were developed as per the manufacturer’s instructions and analyzed using Mabtech IRIS (Mabtech, Stockholm, Sweden) after overnight incubation. To determine class II HLA restriction by ELISpot, CD4+ neo-T cells were first isolated using CD4+ microbeads (Miltenyi) as per manufacturer’s instructions, then incubated for 1 hour at 37°C with 10 μg/mL anti-DP (Abcam, Cambridge, MA), anti-DQ, or anti-DR antibodies (both Biolegend) before incubation with peptides.
Intracellular staining
Neo-T cells were resuspended in T-cell medium at 2 × 106 cells per mL and then stimulated with either neo or irrelevant peptides in the presence of CD28 and CD49d (BD Biosciences), followed by the addition of BD GolgiStop and BD GolgiPlug (BD Biosciences). After overnight incubation, T cells were stained for surface T-cell markers (CD8, CD4, and CD3), washed, permeabilized with BD Cytofix/Cytoperm Solution (BD Biosciences), and then stained with interferon-γ (IFN-γ) and TNF-α antibodies (BD Biosciences). At least 75 000 live cells were acquired using the Gallios flow cytometer, and analysis was performed using Kaluza software.
51Cr-release assay
The cytolytic ability of neo-T cells was assessed in a 6-hour 51Cr-release assay. Target cells were peptide-pulsed or unpulsed OKT3-blasts, tumor cell lines, or primary AML blasts (unmodified or including modifications to express the neoantigen and/or relevant HLA molecules; supplemental Methods). Targets were labeled with 51Cr (PerkinElmer, Waltham, MA), then resuspended with neo-T cells (in triplicate) at various effector-to-target (E:T) ratios, and incubated for 6 hours at 37°C, 5% CO2. Specific lysis was calculated as follows: specific lysis (%) = (experimental – spontaneous release)/(maximum – spontaneous release)×100.
Xenograft model of AML
At 8 to 12 weeks of age, NSG-SGM3 mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(CMV-IL3,CSF2,KITLG)1Eav/MloySzJ, Jackson ImmunoResearch Laboratories Inc, West Grove, PA) were sublethally irradiated (0.8Gy) and then injected via tail vein with 2 × 106 firefly luciferase–labeled Kg1A cells modified to express relevant HLA (B∗35:43) and IDH2R140Q or IDH2wild-type (supplemental Methods). The mice then received IV infusions of neo-T cells on day 1 (2.5 × 107 cells per mouse), day 6 (1.0 × 107 cells per mouse), and day 12 (0.5 × 107 cells per mouse) or phosphate-buffered saline (PBS) only as a negative control. IL-2 (1000 IU) was injected subcutaneously every 3 days, from days 6 to 12. Tumor burden was monitored by bioluminescence imaging using the IVIS Lumina II imaging system and analyzed with Living Image 4.5 software (Caliper Life Sciences, Hopkinton, MA).
Statistical analysis
All statistical analyses were performed using Microsoft Excel version 16 (Microsoft, Redmond, WA) or Prism 9 (GraphPad Software, San Diego, CA). ELISpot means between groups were compared with the Mann-Whitney U test, which provides analysis of paired samples and nonparametric data sets. For in vivo studies, tumor burden between each treatment group was compared using the Student t test.
Results
Detection and characterization of neo-T cells in healthy individuals
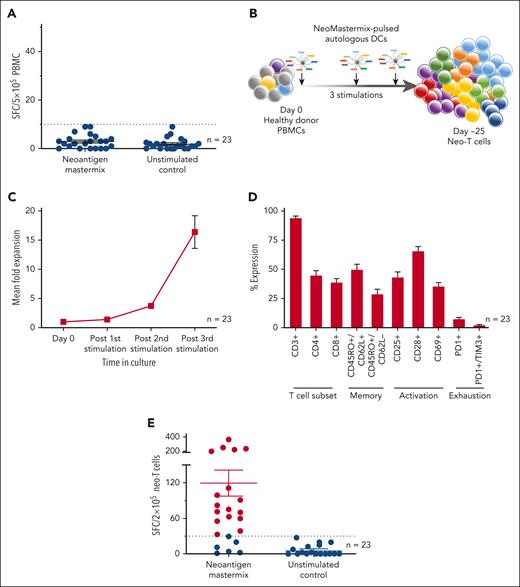
First, to determine whether neo-T cells were present in the circulation, we examined the peripheral blood of 23 healthy donors with diverse HLA types (supplemental Table 1) and measured immune reactivity against candidate neoantigen peptides by IFN-γ ELISpot. As shown in Figure 1A, none of our donors had detectable neo-T cell responses (3 ± 1 spot-forming colonies [SFC] per 5 × 105 PBMCs; mean ± standard error of the mean). To confirm whether this indeed reflected the absence of reactive cells or simply a frequency that was below the threshold of ELISpot detection, we performed in vitro stimulations (Figure 1B) to amplify the frequency of any cells that were present. After 25 days (and 3 stimulations), we achieved a mean 16.4-fold (± 2.8) increase in cell numbers (Figure 1C), with cultures comprised predominantly of T cells (CD3+: 93.8% ± 1.9%) representing both helper (CD4+: 44.6% ± 4.2%) and cytotoxic (CD8+: 38.6% ± 3.4%) subsets (Figure 1D) expressing central (CD45RO+/CD62L+: 49.5% ± 4.9%) and effector memory (CD45RO+/CD62L−: 28.6% ± 4.4%) markers. Furthermore, these expanded cells were activated based on the expression of CD25 (42.9% ± 4.9%), CD28 (65.4% ± 4.1%), and CD69 (35.2% ± 3.4%) with minimal/no expression of exhaustion markers (PD1: 7.1% ± 1.7%, and PD1+/TIM3+: 2.0% ± 0.7%). We then reexamined the neoantigen specificity of these expanded T cells and now detected reactive cells (defined as >30 SFC per 2 × 105 cells) in 74% (n = 17 of 23) of our donors (mean, 120 ± 22 SFC per 2 x 105 cells; Figure 1E). Taken together, these data demonstrate that neo-T cells are present at low levels in the circulation of the majority of healthy individuals and must be amplified to quantitate.
Generation and characterization of neo-T cells derived from healthy donors. (A) Reactivity of PBMCs after exposure to neoantigen mastermix (peptide library encompassing 14 selected AML neoantigens) or medium only (negative control), as measured by IFN-γ ELISpot. Data presented as SFC per 5 × 105 input cells; bars and error bars indicate mean and standard error of mean (SEM), respectively. (B) Schematic of ex vivo expansion protocol. (C) Fold expansion of neo-T cells achieved over 3 stimulations in culture, determined by cell counting based on trypan blue exclusion (n = 23). (D) Immunophenotyping of expanded cells as assessed by flow cytometry, for the expression of T-cell subset, memory, activation, and exhaustion markers (n = 23). (E) Specificity of expanded cells toward the neoantigen mastermix or medium only, as measured by IFN-γ ELISpot (n = 23). Specific cells are defined as ≥30 SFC per 2 × 105 cells (indicated by red dots).
Generation and characterization of neo-T cells derived from healthy donors. (A) Reactivity of PBMCs after exposure to neoantigen mastermix (peptide library encompassing 14 selected AML neoantigens) or medium only (negative control), as measured by IFN-γ ELISpot. Data presented as SFC per 5 × 105 input cells; bars and error bars indicate mean and standard error of mean (SEM), respectively. (B) Schematic of ex vivo expansion protocol. (C) Fold expansion of neo-T cells achieved over 3 stimulations in culture, determined by cell counting based on trypan blue exclusion (n = 23). (D) Immunophenotyping of expanded cells as assessed by flow cytometry, for the expression of T-cell subset, memory, activation, and exhaustion markers (n = 23). (E) Specificity of expanded cells toward the neoantigen mastermix or medium only, as measured by IFN-γ ELISpot (n = 23). Specific cells are defined as ≥30 SFC per 2 × 105 cells (indicated by red dots).
Neo-T cells recognize multiple neoantigens
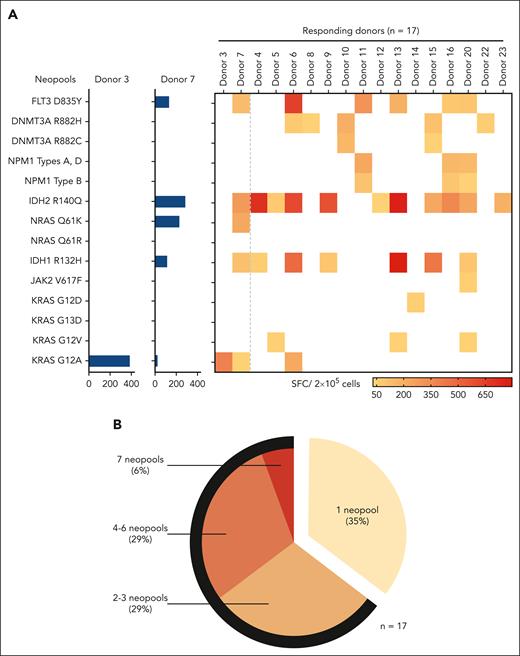
Having established that our pooled neoantigens were immunogenic, we sought to identify whether all elicited T-cell reactivity. Thus, we deconvoluted our mastermix and examined T-cell reactivity against each of the 14 individual stimulating neoantigens separately. Figure 2A (left) shows results from 2 representative donors; in donor 3, individual neoantigen screening revealed a T-cell response entirely focused on the KRASG12A neoantigen (387 SFC per 2 × 105 cells), whereas neo-T cells of donor 7 recognized 5 different neoantigens (IDH2R140Q [286 SFC per 2 × 105 cells], NRASQ61K [229 SFC per 2 × 105 cells], FLT3D835Y [133 SFC per 2 × 105 cells], IDH1R132H [115 SFC per 2 × 105 cells), and KRASG12A [31 SFC per 2 × 105 cells]). Figure 2A (right) summarizes the results for all 17 of our responding donors, whereas Table 1 shows the hierarchy of neoantigen immunodominance (defined as the capacity of each neoantigen to induce IFN-γ from expanded T cells, with ranking based on the number of responding donors and frequency of reactive cells). All but 2 neoantigens (KRASG13D and NRASQ61R) were immunogenic in at least 1 donor. Overall, IDH2R140Q proved to be immunodominant (with the highest number of responding donors [11 of 17, 65%] and magnitude of response [391 ± 92 SFC per 2 × 105 cells]), followed by IDH1R132H (n = 7 of 17 [41%]; 295 ± 106 SFC per 2 x 105 cells) and FLT3D835Y (n = 6 of 17 [35%]; 242 ± 87 SFC per 2 x 105 cells). Overall, 64% of responders recognized ≥2 neoantigens, and more than half of these responders recognized 4 to 7 individual neo-targets (Figure 2B).
Specificity of neo-T cells toward individual neoantigens. (A) Representative example from 2 donors showing IFN-γ ELISpot responses toward each of the 14 neopools individually (left; data presented as SFC per 2 × 105 input cells) and summary data for all donors (right). (B) The percentage of neo-T cell lines showing responses to 1, 2 to 3, 4 to 6, and 7 neopools. The black arc indicates the proportion of multispecific neo-T cells (ie, neo-T cells recognizing ≥2 neoantigens).
Specificity of neo-T cells toward individual neoantigens. (A) Representative example from 2 donors showing IFN-γ ELISpot responses toward each of the 14 neopools individually (left; data presented as SFC per 2 × 105 input cells) and summary data for all donors (right). (B) The percentage of neo-T cell lines showing responses to 1, 2 to 3, 4 to 6, and 7 neopools. The black arc indicates the proportion of multispecific neo-T cells (ie, neo-T cells recognizing ≥2 neoantigens).
Immunodominance of AML neoantigens, ranked by the number of responding donors and IFN-γ ELISpot response
| Mutation . | Responding donors, n (%) . | Mean ± SEM . | Median (range) . |
|---|---|---|---|
| IDH2R140Q | 11 (65%) | 391 ± 92 | 286 (770-31) |
| IDH1R132H | 7 (41%) | 295 ± 106 | 115 (796-58) |
| FLT3D835Y | 6 (35%) | 242 ± 87 | 161.5 (642-85) |
| DNMT3AR882H | 5 (29%) | 69 ± 16 | 64 (111-35) |
| NPM1 types A, D | 3 (18%) | 173 ± 29 | 152 (230-138) |
| NPM1 type B | 3 (18%) | 76 ± 22 | 87 (107-35) |
| KRASG12A | 3 (18%) | 304 ± 84 | 303.5 (387-220) |
| KRASG12V | 3 (18%) | 42 ± 4 | 38 (51-38) |
| DNMT3AR882C | 2 (12%) | 36 ± 0 | 36 (36-36) |
| NRASQ61K | 1 (6%) | 229 ± 0 | 229 (229-229) |
| KRASG12D | 1 (6%) | 55 ± 0 | 55 (55-55) |
| JAK2V617F | 1 (6%) | 45 ± 0 | 45 (45-45) |
| KRASG13D | 0 (0%) | - | - |
| NRASQ61R | 0 (0%) | - | - |
| Mutation . | Responding donors, n (%) . | Mean ± SEM . | Median (range) . |
|---|---|---|---|
| IDH2R140Q | 11 (65%) | 391 ± 92 | 286 (770-31) |
| IDH1R132H | 7 (41%) | 295 ± 106 | 115 (796-58) |
| FLT3D835Y | 6 (35%) | 242 ± 87 | 161.5 (642-85) |
| DNMT3AR882H | 5 (29%) | 69 ± 16 | 64 (111-35) |
| NPM1 types A, D | 3 (18%) | 173 ± 29 | 152 (230-138) |
| NPM1 type B | 3 (18%) | 76 ± 22 | 87 (107-35) |
| KRASG12A | 3 (18%) | 304 ± 84 | 303.5 (387-220) |
| KRASG12V | 3 (18%) | 42 ± 4 | 38 (51-38) |
| DNMT3AR882C | 2 (12%) | 36 ± 0 | 36 (36-36) |
| NRASQ61K | 1 (6%) | 229 ± 0 | 229 (229-229) |
| KRASG12D | 1 (6%) | 55 ± 0 | 55 (55-55) |
| JAK2V617F | 1 (6%) | 45 ± 0 | 45 (45-45) |
| KRASG13D | 0 (0%) | - | - |
| NRASQ61R | 0 (0%) | - | - |
SEM, standard error of the mean.
In-depth characterization of IDH2R140Q-specific neo-T cells
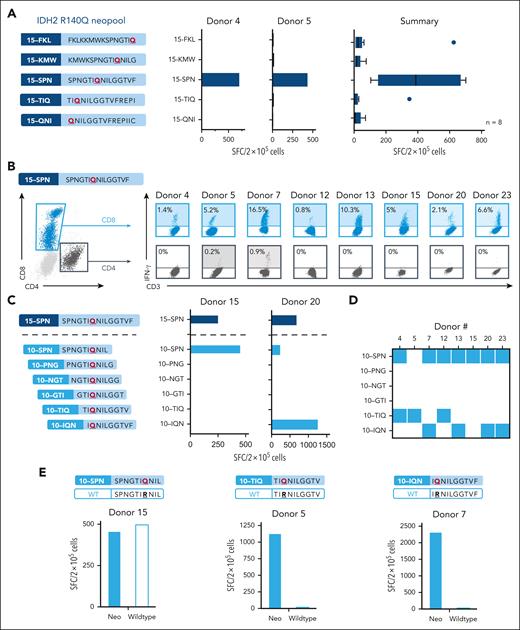
Next, we sought to characterize the profile of the expanded neoantigen-responsive T cells, focusing first on those reactive against the immunodominant IDH2R140Q mutation. To identify the immunogenic 15mer peptide within the neopool, we exposed the neo-T cells to each peptide individually. As shown in Figure 3A for 2 representative (left) and all mapped donors (right, n = 8), the dominant reactivity was directed to the SPN 15mer (SPNGTIQNILGGTVF [15-SPN], AAs 134-148; 403 ± 89 SFC per 2 × 105 cells; n = 8). Given that responsive donors had disparate HLA types, we sought to determine whether there were differences in the recognition profile seen between individuals. We performed intracellular cytokine staining analysis to determine whether reactivity was mediated by CD4+ or CD8+ T cells and, as shown in Figure 3B, we found that all 8 donors tested mounted a CD8+ response (range, 0.8%-16.5%), with 2 donors additionally showing CD4+ reactivity (0.2%-0.9%) above that of unstimulated control (supplemental Figure 2). This demonstrates that the “long” 15-SPN peptide contains both helper (CD4) and cytotoxic (CD8) T-cell epitopes.
In-depth profiling of IDH2R140Q-specific neo-T cells. (A) Deconvolution of the IDH2R140Q response to identify the stimulatory 15mer neopeptide. Schematic showing the 5 overlapping 15mers spanning the IDH2R140Q mutation (left). IFN-γ ELISpot responses toward each 15mer for 2 representative donors (center), and summary results (right, n = 8). Vertical lines for the box indicate the median, 20th, and 80th percentiles. (B) Intracellular cytokine staining analysis to identify reactive CD8 and CD4 subsets recognizing the immunogenic 15-SPN neopeptide based on IFN-γ production. The gating strategy for CD8+ and CD4+ T cells is shown on the left; the percentage of reactive CD8 and/or CD4 cells for individual responders is shown on the right (percentage obtained by subtracting response from unstimulated control). (C) Schematic of 10mers overlapping by 9 AAs that encompass the 15-SPN neopeptide used for minimal epitope mapping (left). Representative example of minimal epitope mapping profiled for neo-T cells from 2 donors (right) and (D) summary of the 8 responding donors, with each colored square indicating the minimal epitope recognized. (E) Analogous IDH2 wild-type peptide sequences generated for each of the IDH2 minimal epitope (top). IFN-γ ELISpot response of neo-T cells after stimulation with the mutant or wild-type peptides for the 10-SPN (left), 10-TIQ (middle), and 10-IQN (right) neoepitopes.
In-depth profiling of IDH2R140Q-specific neo-T cells. (A) Deconvolution of the IDH2R140Q response to identify the stimulatory 15mer neopeptide. Schematic showing the 5 overlapping 15mers spanning the IDH2R140Q mutation (left). IFN-γ ELISpot responses toward each 15mer for 2 representative donors (center), and summary results (right, n = 8). Vertical lines for the box indicate the median, 20th, and 80th percentiles. (B) Intracellular cytokine staining analysis to identify reactive CD8 and CD4 subsets recognizing the immunogenic 15-SPN neopeptide based on IFN-γ production. The gating strategy for CD8+ and CD4+ T cells is shown on the left; the percentage of reactive CD8 and/or CD4 cells for individual responders is shown on the right (percentage obtained by subtracting response from unstimulated control). (C) Schematic of 10mers overlapping by 9 AAs that encompass the 15-SPN neopeptide used for minimal epitope mapping (left). Representative example of minimal epitope mapping profiled for neo-T cells from 2 donors (right) and (D) summary of the 8 responding donors, with each colored square indicating the minimal epitope recognized. (E) Analogous IDH2 wild-type peptide sequences generated for each of the IDH2 minimal epitope (top). IFN-γ ELISpot response of neo-T cells after stimulation with the mutant or wild-type peptides for the 10-SPN (left), 10-TIQ (middle), and 10-IQN (right) neoepitopes.
Given that all of our donors mounted a CD8+ T-cell response, we sought to identify the IDH2R140Q minimal epitope for each responding donor. To do this, we first generated a panel of 10mers (overlapping by 9 AAs) spanning the 15-SPN (Figure 3C, left) and exposed the expanded IDH2R140Q reactive neo-T cell lines to each of these individual peptides. Figure 3C (right) shows the results of 2 representative donors. Donor 15 responded to 15-SPN as well as a single 10mer (10-SPN, SPNGTIQNIL, AAs 134-143; 460 SFC per 2 × 105 cells), whereas donor 20’s neo-T cells recognized 2 distinct 10mers, 10-SPN (232 SFC per 2 × 105 cells) and 10-IQN (IQNILGGTVF, AAs 139-148; 1256 SFC per 2 × 105 cells). The results for all 8 responding donors are shown in Figure 3D. Overall, we identified 3 unique immunogenic minimal CD8+ epitopes within the IDH2R140Q 15-SPN neopeptide: 10-SPN (n = 7 responders), 10-TIQ (TIQNILGGTV, AAs 138-177; n = 3 responders), and 10-IQN (n = 4 responders).
Next, we assessed whether IDH2R140Q-specific neo-T cells demonstrated any wild-type cross-reactivity, an important safety consideration given that the mutant and wild-type peptides differ by a single amino acid, and that wild-type IDH2 is widely expressed in the body, including at high levels in the heart and kidneys. As shown in Figure 3E, although 10-SPN–reactive T cells recognized both the neo- and wild-type peptides at similar levels (460 vs 500 SFC per 2 × 105 cells; left), neo-T cells directed toward 10-TIQ and 10-IQN proved to be highly mutant-specific in all responding donors (10-TIQ: SFC 1070 vs 30 per 2 × 105 cells [center]; 10-IQN: SFC 2300 vs 50 per 2 × 105 cells [right]).
We similarly interrogated the CD4 reactivity detected in donors 5 and 7 by first magnetically selecting CD4+ T cells (supplemental Figure 3A, left), which were then stimulated with the 15-SPN peptide to confirm retention of reactivity (supplemental Figure 3A, right). To ascertain whether these responses were mutant specific, we stimulated reactive cells with either the 15-SPN or wild-type peptide. As shown in supplemental Figure 3B, whereas the CD4+ neo-T cells from donor 5 were unable to discriminate between neopeptides and wild-type peptides (79 and 68 SFC per 2 × 105 cells, respectively), donor 7 recognized only the neopeptide (132 vs 14 SFC per 2 × 105 cells). Hence, we sought to identify the restricting HLA class II allele (donor 7: DR∗04,∗08, DQ∗03,∗04, DP∗04,∗04). As shown in supplemental Figure 3C, 15-SPN–induced IFN-γ was decreased only in the presence of the HLA-DR blocking antibody. Furthermore, these CD4+ neo-T cells specifically lysed peptide-pulsed targets matched at DR∗08 but not DR∗04, indicating that the CD4+ epitope was DR∗08 restricted in this donor (supplemental Figure 3D; Table 2).
Minimal epitopes within the IDH2R140Qneoantigen and associated HLA restriction
| Mutant-specific epitope . | Position . | CD4 or CD8 response . | Responding donors . | HLA restriction . |
|---|---|---|---|---|
| TIQNILGGTV (10-TIQ) | AA 138-147 | CD8 | Donor 5 Donor 12 | B∗15:01 A∗02:06 |
| IQNILGGTVF (10-TIQ) | AA 139-148 | CD8 | Donor 7 Donor 20 | B∗35:43 B∗44:02 |
| SPNGTIQNILGGTVF (15-SPN) | AA 134-148 | CD4 | Donor 7 | DR∗08 |
| Mutant-specific epitope . | Position . | CD4 or CD8 response . | Responding donors . | HLA restriction . |
|---|---|---|---|---|
| TIQNILGGTV (10-TIQ) | AA 138-147 | CD8 | Donor 5 Donor 12 | B∗15:01 A∗02:06 |
| IQNILGGTVF (10-TIQ) | AA 139-148 | CD8 | Donor 7 Donor 20 | B∗35:43 B∗44:02 |
| SPNGTIQNILGGTVF (15-SPN) | AA 134-148 | CD4 | Donor 7 | DR∗08 |
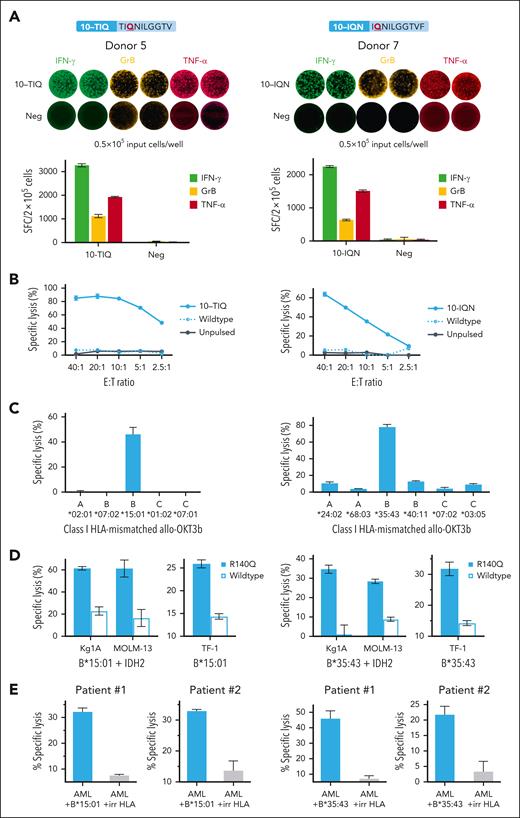
Functional characterization of mutant-specific IDH2R140Q-specific neo-T cells
Given that the in vivo efficacy of infused T cells has closely been linked to their capacity to produce multiple effector molecules (ie, polyfunctionality), we sought to further characterize the functional profile of our neo-T cells using the FluoroSpot assay. We focused on our mutant-specific (ie, 10-TIQ and 10-IQN) T-cell responses, by evaluating reactive cells from donor 5 (TIQ-specific) and donor 7 (IQN-specific) for secretion of effector molecules. As shown in Figure 4A, following exposure to the stimulating neoepitope, TIQ- and IQN-reactive T cells secreted not only IFN-γ (3268 ± 6 SFC per 2 × 105 cells, and 2248 ± 30 SFC per 2 × 105 cells, respectively) but also granzyme B (GrB; 1122.7 ± 69 SFC and 636 ± 26 SFC per 2 × 105) and TNF-α (1929 ± 24 SFC and 1510 ± 37 SFC per 2 × 105) with minimal reactivity in the unstimulated control. As shown in supplemental Figure 4 (left panel), of all IFN-γ–producing TIQ-specific T cells, 54% were polyfunctional; 14%, IFN-γ + GrB; 25%, IFN-γ + TNFα; 15%, IFN-γ + GrB + TNFα. Similarly, for donor 7’s IQN response, 77% of IFN-γ+ cells were polyfunctional (5%, IFN-γ + GrB; 54%, IFN-γ + TNFα; 18%, IFN-γ + GrB + TNFα; supplemental Figure 4, right panel).
Characterization of IDH2R140Q neo-T cells with mutant-specific responses. Neo-T cells directed toward TIQ- and IQN-minimal epitopes were mutant-specific, and further profiled using donor 5 (left column) and donor 7 (right column) as representative examples, respectively. (A) FluoroSpot analysis to assess for IFN-γ, GrB, and TNF-α secretion from neo-T cells (FluoroSpot images, top; corresponding bar graphs, bottom). (B) Reactive neo-T cells specifically lysed autologous neopeptide-pulsed OKT3 blasts, as assessed in a 6-hour 51Cr-release cytotoxicity assay over a range of E:T ratios, from 40:1 to 2.5:1. (C) HLA-restriction studies using allogeneic HLA-mismatched OKT3 blasts transduced with individual class I HLA molecules from the donors as targets. Only neopeptide-pulsed OKT3b expressing B∗15:01 and B∗35:43 were lysed by the TIQ- and IQN-specific neo-T cells, respectively, indicating that these are the restricting HLA alleles (E:T ratio, 40:1). (D) Six-hour 51Cr-release assay showing specific lysis of AML cell lines Kg1A and MOLM13 modified to overexpress IDH2R140Q and the restricting HLA alleles, and R140Q TF-1 that express the neoantigen at native levels (E:T, 60:1). (E) Specific lysis of primary AML blasts from 2 patients modified to express the relevant, or irrelevant HLA molecules (E:T, 60:1).
Characterization of IDH2R140Q neo-T cells with mutant-specific responses. Neo-T cells directed toward TIQ- and IQN-minimal epitopes were mutant-specific, and further profiled using donor 5 (left column) and donor 7 (right column) as representative examples, respectively. (A) FluoroSpot analysis to assess for IFN-γ, GrB, and TNF-α secretion from neo-T cells (FluoroSpot images, top; corresponding bar graphs, bottom). (B) Reactive neo-T cells specifically lysed autologous neopeptide-pulsed OKT3 blasts, as assessed in a 6-hour 51Cr-release cytotoxicity assay over a range of E:T ratios, from 40:1 to 2.5:1. (C) HLA-restriction studies using allogeneic HLA-mismatched OKT3 blasts transduced with individual class I HLA molecules from the donors as targets. Only neopeptide-pulsed OKT3b expressing B∗15:01 and B∗35:43 were lysed by the TIQ- and IQN-specific neo-T cells, respectively, indicating that these are the restricting HLA alleles (E:T ratio, 40:1). (D) Six-hour 51Cr-release assay showing specific lysis of AML cell lines Kg1A and MOLM13 modified to overexpress IDH2R140Q and the restricting HLA alleles, and R140Q TF-1 that express the neoantigen at native levels (E:T, 60:1). (E) Specific lysis of primary AML blasts from 2 patients modified to express the relevant, or irrelevant HLA molecules (E:T, 60:1).
To examine the cytotoxic potential of our cells, we cocultured TIQ- and IQN-reactive cells with autologous peptide-pulsed or unpulsed OKT3 blasts. As shown in Figure 4B, mutant-specific neo-T cells specifically lysed mutant peptide-pulsed OKT3 blasts (10-TIQ: 85.1% ± 3.4% specific lysis; 10-IQN: 63.7% ± 2.2%; E:T ratio, 40:1), with no recognition of wild-type peptide–pulsed or unpulsed targets. To identify the restricting HLA allele(s), we generated a panel of allogeneic targets, each transgeneically expressing single HLA class I alleles from donor 5 (Figure 4C, left) or donor 7 (Figure 4C, right). These were pulsed with the TIQ or IQN peptides and used as targets, which revealed HLA B∗15:01 as the restricting allele for 10-TIQ, and B∗35:43 as the restricting allele for 10-IQN. Table 2 summarizes the results for all mutant-specific responses for which we have performed HLA restriction studies.
Next, we investigated whether neo-T cells could recognize targets expressing endogenous neoantigens. We first assessed this using the AML cell lines Kg1A and MOLM13 that were modified to overexpress the mutant or wild-type IDH2 exon 4 (containing the TIQ and IQN epitope regions; supplemental Figure 5), and the relevant restricting class I HLA alleles (HLA-B∗15:01 and HLA-B∗35:43, respectively). As shown in Figure 4D (left), TIQ-specific neo-T cells specifically lysed IDH2-mutant Kg1AB∗15:01 (61.4% ± 1.6%; E:T ratio, 60:1) and MOLM13B∗15:01 (61.3% ± 7.7%) but not the wild-type counterparts (22.9% ± 3.8% and 16.6% ± 7.7%, respectively). We then extended our analysis to the AML cell line R140Q TF-1, which, because of a knock-in mutation, expresses the IDH2R140Q neoantigen at native rather than overexpressed levels regulated by the endogenous IDH2 promoter. Again, the TIQ-specific T cells specifically lysed R140Q TF-1 modified to express the HLA molecules, with minimal reactivity to the parental TF-1 expressing wild-type IDH2. The findings were similar for IQN-specific neo-T cells (Figure 4D, right). Next, we evaluated the reactivity toward primary AML blasts from 2 patients harboring the IDH2R140Q mutation. Of note, these patients had persistent mutant variant allele frequency of ∼40% despite enasidenib treatment. After the introduction of relevant HLA molecules, mutant-specific T cells specifically lysed malignant blasts in an HLA-restricted manner (Figure 4E). Finally, we cocultured unmanipulated HLA-B∗44-positive primary AML cells with allogeneic HLA-B44–restricted IDH2R140Q-specific neo-T cells, or, as negative controls, HLA-mismatched neo-T cells or virus-specific T cells. As shown in supplemental Figure 6, only HLA-B∗44–restricted neo-T cells exerted specific lysis (35% ± 3%). Taken together, these findings support the capacity of neo-T cells to recognize and target endogenous mutant IDH2R140Q.
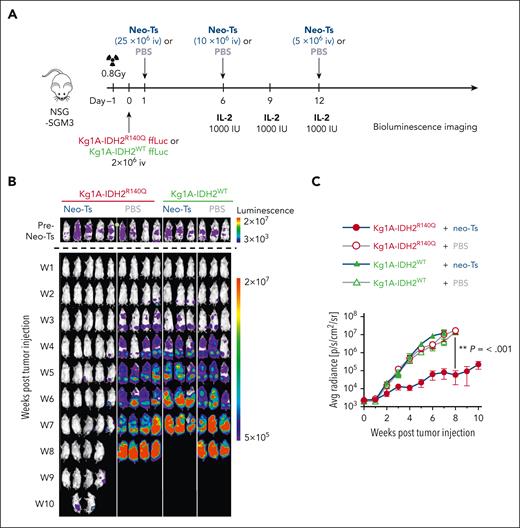
Mutant-specific IDH2R140Q neo-T cells suppress leukemic growth in vivo
To evaluate the antileukemic activity of the IDH2R140-specific neo-T cells in vivo, we engrafted NSG-SGM3 mice with IDH2 mutant or wild-type Kg1A cells modified to express HLA-B∗35:43, then injected neo-T cells on days 1, 6, and 12 (Figure 5A). Tumor growth was monitored by bioluminescence imaging. The tumor burden was similar across all treatment groups before neo-T cell or PBS injections (Figure 5B, top). As shown in Figure 5B (bottom) and C, in mice engrafted with wild-type tumors (Kg1A-IDH2WT), neo-T cells had no antitumor effect, similar to what was observed in PBS-treated control mice. In contrast, Kg1A-IDH2R140Q–engrafted animals treated with neo-T cells showed a significant antitumor effect, compared with in PBS control and wild-type Kg1A–engrafted mice treated with neo-T cells (P < .001).
In vivo activity of neo-T cells. (A) Schematic showing the experimental setup of the Kg1A xenograft model. (B) Bioluminescence imaging showing the tumor burden in each mouse over time. (C) Quantitative analysis of bioluminescence detection in each treatment group.
In vivo activity of neo-T cells. (A) Schematic showing the experimental setup of the Kg1A xenograft model. (B) Bioluminescence imaging showing the tumor burden in each mouse over time. (C) Quantitative analysis of bioluminescence detection in each treatment group.
Discussion
For patients with high-risk AML, the adoptive transfer of tumor-directed donor T cells via allo-HSCT is critical in mediating the graft-versus-leukemia effect to induce complete and durable remissions.21 However, the concurrently transferred alloreactive T cells may damage the patients’ healthy tissues, leading to GVHD, which accounts for 7% to 15% of deaths after allo-HSCT.22 Furthermore, despite the intensive treatment, ∼60% of patients experience disease relapse within 5 years, thus prompting us to explore novel targeted T-cell approaches to minimize GVHD and augment the graft-versus-leukemia effect. In this study, we assessed the immunotherapeutic potential of targeting neoantigens, which arise from malignant cells and are thus exclusive to tumors. As such, the infusion of such cells should provide safe and potent antitumor effects, as has been seen in other diseases. To assess the feasibility of this approach, we explored a range of potential targets including notable driver mutations (eg, DNMT3AR882C/H, IDH2R140Q, and FLT3D835Y), and found that the majority (86%) were immunogenic in donors of diverse HLA types. Furthermore, most individuals responded to multiple neoantigens, highlighting the potential for preparing broad-spectrum T-cell products to address the presence of multiple driver mutations characteristic of AML. Reactive cells arose from both CD4+ and CD8+ T-cell subsets and, importantly, could selectively discriminate mutant from wild-type sequences, thereby supporting the feasibility of clinically implementing such an approach.
Although 12 of 14 target neoantigens proved immunogenic, IDH2R140Q emerged as immunodominant, inducing a response in the majority of donors (11 of 17, 65%) with the highest frequency of reactive cells (391 ± 92 SFC per 2 × 105 cells). IDH2 is a mitochondrial enzyme that converts isocitrate to α-ketoglutarate (αKG), an essential cofactor for several chromatin-modifying enzymes (eg, histone demethylases and the ten-eleven translocation (TET) family of DNA hydrolases including TET1, TET2, and TET3) that are involved in histone and DNA demethylation to promote the myeloid differentiation of hematopoietic stem cells.23 However, in its mutated state, IDH2 reduces αKG to the oncometabolite 2-hydroxyglutarate, which competitively inhibits αKG-dependent enzymes, resulting in differentiation arrest and the accumulation of immature clonal precursor cells.23,24 Clinically, 8% to 15% of de novo AML cases harbor mutations in the IDH2 gene and R140Q is the most frequently detected mutation (in ∼60%-75% of cases).17,24,25 Mutated IDH2 is most frequently seen in patients of older age with AML (median, aged ∼70 years),25 and the mutation has also been found in other diseases including myelodysplastic syndrome, myeloproliferative disorders, cholangiocarcinomas, gliomas, and melanomas, supporting the broader potential of targeting this mutated sequence.26
Currently, enasidenib is the only US Food and Drug Administration–approved targeted therapy for patients with primary IDH2-mutated relapsed/refractory AML. This oral allosteric inhibitor binds to, and deactivates, mutated IDH2, thereby promoting the maturation of myeloid blasts into terminal cells, ultimately inducing clinical remission in 15% to 19% of patients.24 However, drug use can result in severe toxicities including differentiation syndrome, characterized by fluid overload, dyspnea, and hyperbilirubinemia, necessitating early discontinuation and loss of benefit.26 In addition, relapses can arise because of the emergence of additional IDH2 mutations (eg, Q316E and I319M) that disrupt drug binding.25-28 Our proposed therapeutic approach involves targeting IDH2R140Q using adoptively transferred neo-T cells, which should kill rather than induce differentiation of mutant-harboring malignant blasts. Because this neoantigen is selectively expressed by the tumor with no normal tissue expression, the risk of “on-target, off-tumor” effects is minimal. Furthermore, donor-derived therapeutic T cells have the capacity to persist long-term to sustain antileukemic benefits in vivo. 27
One potential risk when targeting a heterogeneous tumor with a monotargeted therapy is immune escape. Indeed, for patients who receive CD19 CAR T cells for relapsed/refractory B-cell acute lymphoblastic leukemia, 7% to 33% of patients will relapse with CD19− disease.28-30 However, as demonstrated in our previous clinical trials, it is possible to prepare and infuse T cells targeting multiple tumor-expressed antigens.13,31,32 For example, in the setting of high-risk/relapsed AML, our group prepared donor-derived T-cell lines targeting the WT1, survivin, PRAME, and NY-ESO-1, which were infused to patients after allo-HSCT.13 The infusions (at doses ranging from 0.5 × 107/m2 to 10 × 107/m2) were safe and associated with benefit, prolonging leukemia-free and overall survival in patients with high-risk patients disease who received prophylactic infusion; and resulting in 1 complete and 1 partial response in 8 patients who received infusions for active relapsed disease. In this study, we demonstrate that multiple neoantigens can also be simultaneously targeted, which may be particularly clinically meaningful given that there are an average of 13 somatic mutations per patient AML sample.33 In fact, 9 of 11 IDH2R140Q-specific neo-T cell lines that we generated (using the neo-peptide pool) also recognized other targets, including IDH1R132H (n = 7), FLT3D835Y (n = 5), KRAS (n = 5), and NRAS (n = 1), establishing the feasibility of multineo (and even combining neotargeting and cancer testes antigen targeting) to provide a targeted therapy with broad coverage to effectively treat this heterogeneous disease.
Although our current work has focused on harnessing ex vivo expanded T cells to target neoantigens, isolated and transgeneically expressed TCRs can also be exploited for therapeutic purposes.34,35 Several early-phase clinical trials have evaluated the therapeutic potential of TCR-modified T cells.15,36-38 For instance, in the setting of high-risk AML, Chapuis et al prophylactically administered WT1-directed A∗02:01-restricted TCR T cells to 12 patients and all remained relapse-free at a median follow-up of 44 months (compared with 54% relapse-free survival in a concurrent comparative cohort).36 Tawara et al used WT1-directed HLA-A∗24:02–restricted TCR T cells to treat active disease, and of the 8 patients who received infusion, 2 showed a transient decrease in leukemic blast counts.15 As such, harnessing neoantigen-targeted TCRs for transgenic modification may also provide therapeutic benefit to patients, given that such cells can be rapidly prepared (1-2 weeks in vitro culture) and thus either generated on demand or prospectively banked for patients with high-risk disease, of the appropriate HLA profile. Furthermore, the transgenic TCR itself can be selected from potent naturally occurring clones, and/or further modified to enhance therapeutic potency or in vivo persistence.34 For instance, Rosenberg et al used a medium affinity (1987 pg/mL IFN-γ after peptide exposure) and high affinity (17 161 pg/mL) TCR targeting the same MART1 epitope, which resulted in partial responses in 4 of 31 (13%) vs 6 of 20 (30%) patients who received treatment.39 TCR affinity can be artificially enhanced, for example by modifying the complementarity-determining region to increase TCR-peptide/major histocompatibility complex binding. For example, Robbins et al modified an HLA-A∗02:01–restricted NY-ESO-1–specific TCR with 2 complementarity-determining region 3α amino acid substitutions (threonine→leucine and serine→tyrosine), resulting in enhanced specific lysis of A∗02:01+/NY-ESO-1+ tumor targets by ∼20% in vitro.40 When applied clinically to patients with metastatic melanoma, 11 of 20 patients achieved durable objective clinical responses including 4 clinical remissions. So the native TCR can be harnessed and also further modified for optimal benefit.41 Finally, tebentafusp is the first US Food and Drug Administration–approved TCR-based engager molecule targeting an HLA-A02–restricted epitope of the melanoma antigen gp-100 that has demonstrated a survival advantage in a randomized trial of patients with metastatic or treatment refractory melanoma, demonstrating the importance of discovering individual TCRs specific for tumor cells.42
In summary, we have demonstrated the immunogenicity of neoantigens arising from recurrent mutations in AML, and the feasibility of generating ex vivo expanded endogenous T cells from healthy donors that target multiple neoantigens simultaneously and that can discriminate between malignant and wild-type sequences. Taken together, these data provide the rationale for future clinical translation and using in vitro expanded neo-T cells to prevent and/or treat relapsed/refractory disease in patients with high-risk AML.
Acknowledgments
The authors thank Walter Mejia for artwork and formatting of manuscript figures.
A.M.L. is supported by a Specialized Center of Research Award from the Leukemia & Lymphoma Society (7019-19), and the National Cancer Institute, National Institutes of Health Lymphoma SPORE (P50 CA126752). P.L. is supported by the Cancer Prevention and Research Institute of Texas Early Career Investigator Award (ECIA, RP200584), Edward P. Evans Foundation MDS Discovery Research Grant, and The Frank Stahl Donation. P.S. is supported by the Center for Cell and Gene Therapy Research Training Program T32 (HL092332). V.H. is supported by the Cancer Prevention and Research Institute of Texas (RR170024). W.K.L. is supported by the Cancer Research Trust New Zealand Murray Jackson Clinical Fellowship, and HSANZ New Investigator Scholarship.
Authorship
Contribution: W.K.L. designed the research, performed research, analyzed data, and wrote the paper; P.S. analyzed the data and edited the manuscript; A.G.T.C., M.F.-K., M.M., L.C.H., M.K., Y.V., and A.W. performed research; N.W. analyzed data; V.H. analyzed data and edited the manuscript; P.L. designed the research, analyzed data, and edited manuscript; and A.M.L. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: A.M.L. is a cofounder, equity holder, and consultant for AlloVir. L.C.H. is a consultant for March Biosciences, and serves on the speakers bureau for Kite. M.K. is a consultant to Allovir. M.M. has received research grants from Fate Therapeutics; consulting fees from March Biosciences and Galapagos NV; travel grants/honoraria from Fate Therapeutics, Amgen, and Galapagos NV; licensing fees and royalties from Fate Therapeutics, Allogene Therapeutics, and March Biosciences; serves on the advisory board for March Biosciences and NKILT Therapeutics; and has equity in March Biosciences. N.W. received licensing fees and royalties from Fate Therapeutics. P.L. received clinical trial funding from Bristol Myers Squib, Allovir, and Marker Therapeutics. V.H. holds stock in Marker Therapeutics and AlloVir; and is a consultant to AstraZeneca. Y.V. is a consultant to Allovir. The remaining authors declare no competing financial interests.
Correspondence: Wingchi K. Leung, Baylor College of Medicine, 1102 Bates Ave, Houston, TX 77030; email: wingchi.leung@bcm.edu.
References
Author notes
A.G.T.C. and M.F.-K. are joint second authors.
Data are available on request from the corresponding author, Wingchi K. Leung (wingchi.leung@bcm.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal