In this issue of Blood, Quaranta et al identify human circulating hematopoietic stem and progenitor cells (cHSPCs) that help maintain steady-state hematopoiesis, which are primed for activation and impact clonal redistribution within the bone marrow.1 cHSPCs and their egress mechanism out of the bone marrow are fairly well characterized in mice, where they act as immunosurveillance units, contributing to the extramedullary production of immune cells.2-5 Because of technical limitations, cHSPCs are understudied in humans, and our knowledge is limited to a few studies showing correlation of the frequency of this population with different physiological and pathologic conditions.6-8 Better understanding these progenitors could provide key insights to improve the current hematopoietic progenitor mobilization protocols, positively impacting in the therapeutic collection and use of these cells. Quaranta et al perform a thorough phenotypic, molecular, and functional characterization of human cHSPCs, as well as an examination of the interplay between this population and their bone marrow resident counterparts.
The authors found that cHSPC frequency decreases with age and is altered in pathologic hematopoiesis (ie, Wiskott-Aldrich syndrome, adenosine deaminase deficiency with severe combined immunodeficiency, thalassemia, and bone marrow failure syndrome), mostly mirroring bone marrow hematopoietic stem and progenitor cell (HSPC) changes. Furthermore, in healthy individuals, the immunophenotypic composition of bone marrow HSPCs differ from cHSPCs, with the latter enriched for multipotent progenitors and multilymphoid progenitors (MLPs), suggesting a distinct role for these populations.
An important contribution in this study from Quaranta et al is the molecular profile of this population. The authors performed a combination of immunophenotyping and single-cell transcriptomics (cellular indexing of transcriptomes and epitopes), revealing, for the first time, to our knowledge, the expression profile of these circulating progenitors. This set of data has value by itself, as it provides the molecular factors that define this population. Indeed, a molecular signature identified in cHSPCs suggested they are in a preactivated state (ie, lower dormancy and primed for differentiation) compared with bone marrow progenitors. These transcriptional findings were functionally confirmed, as cHSPCs have faster repopulating dynamics and limited self-renewal potential after transplant. These results support the proposed role for cHSPCs as quick responders for differentiation in case of demand.
As expected, cHSPCs express lower levels of bone marrow retention molecules. CXCR4, for example, has the biggest expression difference compared with bone marrow HSPCs. This is remarkable as CXCR4 critically participates in HSPC cell cycle control9 and suggests a coordinated regulation between dormancy/activation and bone marrow egress.
Additionally, to gain insights into the migratory fate of these cHSPCs, Quaranta et al analyzed the transcriptional profile of hematopoietic progenitors found in different tissues (ie, spleen, thymus, lung, liver, kidney, lymph nodes, and bone marrow), creating human organ–resident (HuOR) HSPC signatures. Importantly, HuOR bone marrow is enriched in the most primitive cHSPC subpopulations, supporting their bone marrow origin. All the extramedullary organ signatures are reflected to a different degree in multiple cHSPC subpopulations. The link between circulating MLPs and the HuOR-thymus signature is noteworthy in that it is better represented in circulating MLPs than in bone marrow MLPs, suggesting the role of circulating MLPs seeding the thymus and contributing to T-cell production. The higher in vitro T-cell differentiation capability of circulating MLPs, compared with those from the bone marrow, supports these conclusions. These findings have interesting implications for our understanding of the reduction of thymic activity during aging,10 as the reduction of thymic-primed circulating MLPs with age could contribute to this process.
Finally, Quaranta et al dig into an important concept. Do cHSPCs efficiently migrate to other bone marrow areas, and what are the consequences? To solve this question, the authors take advantage of the vector integration in HSPCs treated with gene therapy. With this approach, they confirm some of their previously described findings (eg, cHSPCs have their origin in the bone marrow, and circulating MLPs contribute more to T cells than bone marrow MLPs), and expand some of them. Integration sites were more commonly shared between cHSPC and lymphoid bone marrow progenitors, emphasizing the higher tendency of this subpopulation to enter circulation. More importantly, the clonal tracking of these integration sites in different bone marrow areas corroborates that a fraction of cHSPCs reenter the bone marrow. This fraction corresponds with the most primitive cHSPCs, which might have implications in the clonal redistribution of progenitors in the bone marrow.
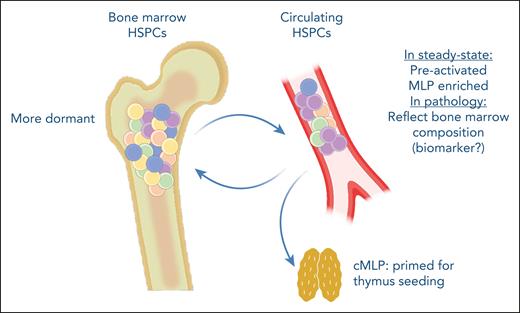
In summary, Quaranta et al identify cHSPCs as active contributors to hematopoiesis with a preactivated state that allows them to quickly differentiate to cover tissue-specific or systemic needs (see figure). Furthermore, their molecular characterization should propel future studies identifying HSPC homing and mobilization mechanisms, potentially impacting the therapeutic use of these progenitors.
cHSPCs are preactivated and represent a quick response progenitor population, contributing to extramedullary hematopoiesis. They are enriched for multilymphoid progenitors, which are transcriptionally programmed to preferentially migrate to the thymus. Furthermore, cHSPCs can reenter the bone marrow, potentially contributing to clonal redistribution. Additionally, cHSPCs could have a use as biomarkers of the bone marrow composition in hematopoietic pathologies. cMLP, circulating multilymphoid progenitor.
cHSPCs are preactivated and represent a quick response progenitor population, contributing to extramedullary hematopoiesis. They are enriched for multilymphoid progenitors, which are transcriptionally programmed to preferentially migrate to the thymus. Furthermore, cHSPCs can reenter the bone marrow, potentially contributing to clonal redistribution. Additionally, cHSPCs could have a use as biomarkers of the bone marrow composition in hematopoietic pathologies. cMLP, circulating multilymphoid progenitor.
Conflict-of-interest disclosure: A.M.-H. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal