In this issue of Blood, Kumar et al use a model antibody to show that a subpopulation of antiprothrombin antibodies found in people with antiphospholipid syndrome act as anticoagulants by stabilizing prothrombin into a conformation that limits its activation by the prothrombinase complex.1
Diagnosis of the rare autoimmune disorder antiphospholipid syndrome requires the persistent presence of antiphospholipid antibodies in people with thrombosis or pregnancy-associated morbidity. Thrombotic risk is estimated with antibody profiles based on 3 different antiphospholipid antibodies: antibodies that cause prolongation of clotting time in sensitive clotting assays, so-called lupus anticoagulant, antibodies against the plasma protein β2-glycoprotein I, and antibodies against the phospholipid cardiolipin.2 Of these 3 antiphospholipid antibodies, only lupus anticoagulant is independently associated with increased risk of thrombosis or pregnancy morbidity. Thrombotic risk is highest in people who are triple positive (ie, positive for anti–β2-glycoprotein I and anti-cardiolipin antibodies as well as lupus anticoagulant).2 Antibodies against the complex of the coagulation factor prothrombin (PT) and the phospholipid phosphatidylserine (PS) (anti-PS/PT antibodies) are frequently found in people with antiphospholipid syndrome. Although the relationship between antiprothrombin antibodies and lupus anticoagulant has been known for decades, their contribution to thrombotic risk is unclear. As a result, they are currently not used in antiphospholipid syndrome diagnostics or risk assessment. However, as most triple-positive people with antiphospholipid syndrome also have antiprothrombin antibodies, they currently receive renewed attention.3
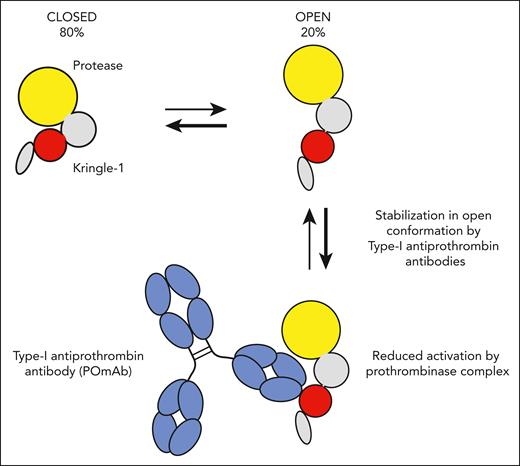
Previous work by the authors showed that anti-PS/PT antibodies can be divided into 2 distinct groups based on functional properties. Type I antibodies interact with the kringle-1 domain of prothrombin, mildly prolong clotting time, and cause accumulation of prothrombin on phospholipid surfaces. In contrast, type II antibodies bind the phospholipid-binding γ-carboxyglutamic acid–rich (GLA) domain of prothrombin and limit accumulation of prothrombin on phospholipid surfaces.4 Here, Kumar et al use a set of elegant experiments to delineate the mechanism of action of type I antiprothrombin antibodies. Prothrombin was previously shown to circulate in equilibrium between 2 conformations: the predominant “closed” conformation that is fully susceptible to activation by prothrombinase and the less frequently occurring “open” conformation with reduced susceptibility to activation (see figure).5 Using fluorescence resonance energy transfer and surface plasmon resonance analysis, the authors show that the model type I anti-prothrombin antibody POmAb (prothrombin open monoclonal antibody) selectively binds the open conformation of prothrombin and, importantly, forces it to remain in this conformation. Structural insights obtained with cryogenic electron microscopy indicate POmAb effectively pushes the protease domain out of the way, thus stabilizing the open conformation and providing an explanation for the inhibitory effects of POmAb on prothrombin activation and clotting of plasma. The observation that other type I antiprothrombin antibodies behave similarly strengthens these findings.
Prothrombin circulates in equilibrium between closed and open conformations. Under normal conditions, ≈80% of all prothrombin is in the closed conformation, in which the kringle-1 domain (red) interacts with the protease domain (yellow). Type I antiprothrombin antibodies found in people with antiphospholipid syndrome recognize an epitope on the kringle-1 domain of prothrombin that is only exposed in the open conformation of prothrombin. Binding of model type I antiprothrombin antibody POmAb (prothrombin open monoclonal antibody) to kringle-1 stabilizes the open conformation, resulting in reduced conversion to thrombin by prothrombinase.
Prothrombin circulates in equilibrium between closed and open conformations. Under normal conditions, ≈80% of all prothrombin is in the closed conformation, in which the kringle-1 domain (red) interacts with the protease domain (yellow). Type I antiprothrombin antibodies found in people with antiphospholipid syndrome recognize an epitope on the kringle-1 domain of prothrombin that is only exposed in the open conformation of prothrombin. Binding of model type I antiprothrombin antibody POmAb (prothrombin open monoclonal antibody) to kringle-1 stabilizes the open conformation, resulting in reduced conversion to thrombin by prothrombinase.
This work has 2 implications. First, Kumar et al provide evidence that anti-prothrombin antibodies can act as true anticoagulants. This important distinction will allow researchers to refine the currently existing antibody profiles in antiphospholipid syndrome for risk stratification, thus bringing us a step closer to accurate identification of patients at risk of (recurrent) thrombosis and pregnancy complications. Nevertheless, although the evidence to support the anticoagulant effects of type I antiprothrombin antibodies is compelling, antiprothrombin antibodies have also been linked to both platelet activation and activated protein C resistance.6-8 Whether type I anti-prothrombin antibodies thus have a net prothrombotic rather than a protective anticoagulant effect remains to be determined. Second, this work will open new avenues to explore the use of antibodies, or fragments thereof, that stabilize prothrombin in its open conformation as anticoagulants. Any clinical applications of type I antiprothrombin antibodies as anticoagulants will require the absolute certainty that they do not have prothrombotic effects.
Conflict-of-interest disclosure: R.T.U. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal