Loss-of-function variants generating premature termination codons associate with bleeds; RNA levels differ between replicate cell cultures.
No PTC-truncated proteins were detected; ENG Q436X (but not R93X) induced cell stress and had pathogenic AlphaMissense readthrough scores.
Visual Abstract
For monogenic diseases caused by pathogenic loss-of-function DNA variants, attention focuses on dysregulated gene-specific pathways, usually considering molecular subtypes together within causal genes. To better understand phenotypic variability in hereditary hemorrhagic telangiectasia (HHT), we subcategorized pathogenic DNA variants in ENG/endoglin, ACVRL1/ALK1, and SMAD4 if they generated premature termination codons (PTCs) subject to nonsense-mediated decay. In 3 patient cohorts, a PTC-based classification system explained some previously puzzling hemorrhage variability. In blood outgrowth endothelial cells (BOECs) derived from patients with ACVRL1+/PTC, ENG+/PTC, and SMAD4+/PTC genotypes, PTC-containing RNA transcripts persisted at low levels (8%-23% expected, varying between replicate cultures); genes differentially expressed to Bonferroni P < .05 in HHT+/PTC BOECs clustered significantly only to generic protein terms (isopeptide-bond/ubiquitin-like conjugation) and pulse-chase experiments detected subtle protein maturation differences but no evidence for PTC-truncated protein. BOECs displaying highest PTC persistence were discriminated in unsupervised hierarchical clustering of near-invariant housekeeper genes, with patterns compatible with higher cellular stress in BOECs with >11% PTC persistence. To test directionality, we used a HeLa reporter system to detect induction of activating transcription factor 4 (ATF4), which controls expression of stress-adaptive genes, and showed that ENG Q436X but not ENG R93X directly induced ATF4. AlphaFold accurately modeled relevant ENG domains, with AlphaMissense suggesting that readthrough substitutions would be benign for ENG R93X and other less rare ENG nonsense variants but more damaging for Q436X. We conclude that PTCs should be distinguished from other loss-of-function variants, PTC transcript levels increase in stressed cells, and readthrough proteins and mechanisms provide promising research avenues.
Introduction
Phenotypic predictions are difficult for hereditary hemorrhagic telangiectasia (HHT), which is an example of many monogenic conditions in which the single disease-causing allele does not explain severity differences between individuals or within the same individual over a lifetime.1 HHT is typically caused by a heterozygous, loss-of-function variant in ACVRL1, ENG, or SMAD4,2 each of which encodes an endothelial cell–expressed protein that transmits or regulates signaling by the bone morphogenetic protein (BMP)/transforming growth factor β (TGF-β) superfamily.3,4 Only a small percentage of cells (mean, 1.7%) lose the second wild-type allele,5 and in abnormal ENG+/− vessels, approximately half normal endoglin (ENG) protein expression remains.6,7 Loss-of-function leads to the generation of abnormal blood vessels, as confirmed by heterozygous and homozygous models.8-15 However, patient-derived HHT endothelial cells display compensatory regulatory mechanisms16-20 so successfully that, in 2-dimensional culture, they behave functionally indistinguishably from isogenic controls, with differences unmasked only when grown in 3-dimensional organ-on-chip devices.20
For patients with HHT, the main hematological problem is anemia due to recurrent bleeds from nasal and gastrointestinal telangiectasia.21,22 Most people with HHT experience nosebleeds sufficient to lead to iron deficiency anemia unless iron intake is supplemented,23 and there can be extreme health care burdens for patients when nasal and/or gastrointestinal HHT bleeding is severe, because they need regular intravenous iron, red cell transfusions, interventional treatments, and/or disease-modifying drugs such as antiangiogenic agents to treat bleeding.24-26 Clinical research platforms suggest “environmental” risk factors for more severe HHT manifestations including iron deficiency,27-33 iron treatments,29,30,32,34,35 and blood-borne infections35,36 although these have not been tested in animal models. By genotype, hemorrhagic severity is greater in patients with HHT who by chance, also have rare deleterious variants in genes that cause general population bleeding disorders37 but hemorrhagic severity does not differ according to the HHT-causal ACVRL1+/− or ENG+/− mutation.2,37
We hypothesized that the precise pathogenic variant may explain some of the variability. HHT-causal variants are usually frameshift or nonsense mutations that result in premature termination codons (PTCs).38 PTC terminology is based on complementary DNA (cDNA) expression constructs, which generate truncated proteins; however, in native cells, transcriptome-wide studies indicate that most PTC-containing transcripts are degraded39,40 by cellular safeguarding processes including nonsense-mediated decay (NMD) that is intron and translation dependent.39-43 NMD efficiency predictions43 and clinical guidance44 incorporate NMD exceptions predicted by PTC location (for example, <50-55 nucleotides [nts] upstream of last exon junction; <150 nts from translational start, or >400 nts–long exons).39,40 NMD efficiency is lower for RNAs with half-lives of <1 hour.39 It is not known why proportions of PTC transcripts escaping NMD differ by tissue/individual,39,40 nor why PTC transcripts are more likely to persist if the population allele frequency is higher,40 or if the PTC is in a coding transcript.40
In cells derived from patients with HHT, evidence supports both PTC RNA loss45 and persistence, generating short-lived, endoplasmic reticulum-retained proteins,46-51 but there has been no attention to how PTC-specific processes may operate alongside pathway-specific perturbations in the heterozygous state. Here, we present evidence demonstrating additional consequences to disease-causing loss-of-function.
Methods
Details are provided in supplemental Material, available on the Blood website.
Clinical HHT research populations
This research was approved by national ethics committees. Three overlapping subpopulations were examined. Two previously described cohorts had extensive hemorrhage phenotyping before genotyping through clinical37 or research programs.2 The third was a previously unreported early HHT population treated by nasal ointments.
Endothelial cell methods
This research was approved by national ethics committees, and all human participants provided written informed consent. Primary studies were performed in blood outgrowth endothelial cells (BOECs) established from healthy volunteers and pregenotyped patients with HHT, using previously optimized methods.52
For RNA sequencing, RNA was extracted from 16 separate cultures of confluent BOECs from 4 separate control and HHT donors and cultured in the presence and absence of BMP9 at 10 ng/mL for 1 hour. Library preparations, genome alignments, and initial normalizations and variant calls53 were performed fully blinded by Genewiz (Leipzig, Germany).
For single-cell quantitative reverse transcription polymerase chain reaction (scqRT-PCR,54,55) 40 viable (DRAQ7 negative) single cells were sorted directly into 96-well plates containing pre-amplification mix for 48 genes selected either as endothelial gene markers, negative controls, or for HHT relevance (supplemental Table 1).
Metabolic labeling was performed in BOEC protein extracts using ENG monoclonal antibody (mAb) SN6h (Dako Denmark A/S, Denmark), with methods modified from Pece et al.46 ImageJ software56 was used for densitometry analyses: background-adjusted values were expressed relative to the start of the chase (0 hours for immature ENG), or to background-adjusted values after 2 hours of chase (for mature ENG).
RNA sequencing analyses
Percentage loss of the nonsense allele was calculated assuming that without NMD,39-43 there would have been no allelic imbalance. Across 16 807 Ensembl57 gene identifiers, the intra-assay coefficient of variation (CV)58 was calculated for replicate cultures, and stringent analyses restricted to genes in which all 4 untreated replicate pairs had a CV of <10% (“met CV10”). Housekeeper genes for DeSeq2 normalization59,60 were the least variable of human transcripts identified in transcriptome data sets from tissues and cell lines (defining inequalities of distribution by the GINI coefficient more commonly used in economics [supplemental Table 2]).61,62 Pathway enrichment analysis for Gene Ontology63 and Uniprot Key Words64 was performed for differentially expressed genes using Functional Annotation Clustering through the Database for Annotation, Visualization, and Integrated Discovery65 version 6.8.
ISR expression system
Wider ENG variant considerations and modeling
For all ENG nonsense variants in the HHT mutation database downloaded in 2018,2,68 we derived individual stop codon sequences (TGA/TAA/TAG) from cDNA descriptors using gnomAD 4.0.069 and GRCh38/hg3870/University of California, Santa Cruz (UCSC) Genome Browser.71 Domains were assigned to these ENG nonsense variants, based on the crystallographic structures of the orphan region (OR) and zona pellucida (ZP) module of human ENG.72 AlphaFold73 models of the OR2 and ZP-N domains of ENG (corresponding to residues S47-R199 and T349-L443, respectively), were extracted from predictions of its complete ectodomain, generated using a local copy of AlphaFold installed using version 2.3.2 of the open-source code available at https://github.com/deepmind/alphafold. AlphaMissense74 predictions for the 19 potential amino acid substitutions that may, in principle, be introduced upon readthrough of each ENG nonsense codon were obtained from file AlphaMissense_aa_substitutions.tsv (version 19 September 2023, 2:02:53 PM), downloaded from https://zenodo.org/records/10813168. Structural figures were generated using PyMOL (Schrödinger, LLC).
Data analysis
STATA IC version 15.0 (Statacorp, College Station, TX) and GraphPad Prism 9 (GraphPad Software, San Diego, CA) were used to calculate summary statistics; compare groups using the Kruskal-Wallis test (Dunn's test was used to derive pairwise comparisons); perform 2-way analysis of variance, linear regression, unsupervised hierarchical clustering, and principal component analyses; and for visualization.
Results
Genotype–phenotype associations with PTCs
To explore whether there might be phenotypic differences for patients with PTC-generating causal variants, we examined unexplained, variable phenotypes and compared patients with obligate PTC-generating causal variants (nonsense; frameshift) with those with non–PTC-generating causal variants (full gene deletions; start codon loss, missense, inframe indels, and pathogenic 5′ untranslated region [UTR] variants).
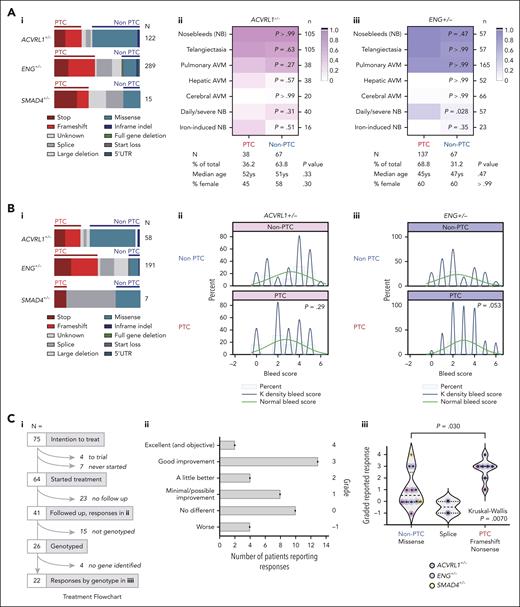
Two cohorts who had undergone formalized hemorrhage phenotyping before genotyping2,37 had similar PTC/non-PTC variant distributions across the HHT genes (Figure 1Ai,Bi). As shown previously, ACVRL1/ENG genotypes were not associated with differing prevalence of nosebleeds.2,37 However, in the first cohort (Figure 1Aii-iii), severe nosebleeds, as defined by Joyce et al,37 were 3.3-fold more common in patients with ENG PTCs than those with ENG non-PTC causal variants (P = .028; Figure 1Aiii). In the second cohort,2 formalized 7-point bleeding scores were similar in patients with ACVRL1 PTC or non-PTC variants (Figure 1Bii) but higher in patients with ENG PTCs (mean, 2.41) compared to ENG non-PTC causal variants (mean, 3.01; P = .05; Figure 1Biii). We also tested PTC associations in a third cohort with an unexplained variable response to topical nasal ointments used by otorhinolaryngologists to accelerate the healing of scabs by reducing ongoing cellular injury and inflammation (Figure 1Ci). As detailed in Figure 1Cii, reported responses had varied markedly with no apparent explanation by demographics, or on identification of HHT causal mutations several years later. The new categories showed that patients with obligate PTC-generating variants were more likely to report beneficial responses than patients with non–PTC-generating missense variants (P = .0070; Figure 1Ciii), and this was most apparent for ENG (P = .054; Figure 1Ciii). Taken together, the data supported phenotypic distinctions between patients with HHT with PTC- and non–PTC-generating causal variants.
PTC clinical correlations in HHT cohorts. (A) Cohort 1 population first described by Joyce et al.37 (i) Molecular subtypes in the 3 endothelial-expressed HHT genes. Note splice variants and multiexon deletions do not enable PTC-generating consequences to be predicted38 and were not examined further. (ii/iii) HHT phenotypes in patients with (ii) ACVRL1+/− and (iii) ENG+/− according to PTC ∗(red) and non-PTC (blue) categories, with exact molecular subtype, color scheme as indicated. Note that throughout the figures, for consistency, ACVRL1+/− is pink/purple; ENG+/− is blue, and SMAD4+/− is orange. P values calculated by Mann-Whitney U test. (B) Cohort 2 population, first described with bleeding scores by Shovlin et al2 (see also supplemental Methods). (i) Molecular subtypes annotated as in panel Ai. (ii/iii) Histograms of bleeding scores with superimposed normal and Kernal cosine distributions, for patients with heterozygous (ii) ACVRL1 and (iii) ENG causal variants. PTC/non-PTC P values calculated by Mann-Whitney U test. (C) Cohort 3: newly described nosebleed ointment cohort (further details provided in supplemental Figure 1). (i) Flowchart of treatment numbers 09/04/1999 to 17/03/2005; patients reporting a response; and patients genotyped. (ii) All 41 reported responses by qualitative description and applied grade. (iii) All 22 reported responses in patients with a causal HHT gene variant identified, by variant type and causal gene (pink, ACVRL1; blue, ENG; and orange, SMAD4). Splice variants are shown separately because RNA consequences are unclear38 and differed when tested in different members of the same family (C. L. Shovlin, unpublished data, 2012). Pairwise P value calculated by Dunn's test after Kruskal-Wallis test. AVM, arteriovenous malformation; NB, nosebleed.
PTC clinical correlations in HHT cohorts. (A) Cohort 1 population first described by Joyce et al.37 (i) Molecular subtypes in the 3 endothelial-expressed HHT genes. Note splice variants and multiexon deletions do not enable PTC-generating consequences to be predicted38 and were not examined further. (ii/iii) HHT phenotypes in patients with (ii) ACVRL1+/− and (iii) ENG+/− according to PTC ∗(red) and non-PTC (blue) categories, with exact molecular subtype, color scheme as indicated. Note that throughout the figures, for consistency, ACVRL1+/− is pink/purple; ENG+/− is blue, and SMAD4+/− is orange. P values calculated by Mann-Whitney U test. (B) Cohort 2 population, first described with bleeding scores by Shovlin et al2 (see also supplemental Methods). (i) Molecular subtypes annotated as in panel Ai. (ii/iii) Histograms of bleeding scores with superimposed normal and Kernal cosine distributions, for patients with heterozygous (ii) ACVRL1 and (iii) ENG causal variants. PTC/non-PTC P values calculated by Mann-Whitney U test. (C) Cohort 3: newly described nosebleed ointment cohort (further details provided in supplemental Figure 1). (i) Flowchart of treatment numbers 09/04/1999 to 17/03/2005; patients reporting a response; and patients genotyped. (ii) All 41 reported responses by qualitative description and applied grade. (iii) All 22 reported responses in patients with a causal HHT gene variant identified, by variant type and causal gene (pink, ACVRL1; blue, ENG; and orange, SMAD4). Splice variants are shown separately because RNA consequences are unclear38 and differed when tested in different members of the same family (C. L. Shovlin, unpublished data, 2012). Pairwise P value calculated by Dunn's test after Kruskal-Wallis test. AVM, arteriovenous malformation; NB, nosebleed.
Establishing BOEC platforms
To examine native consequences of PTC-generating variants, we cultured primary BOECs from individuals with known disease-causing nonsense variants (patients with HHT) and healthy volunteers. As shown in supplemental Figure 2, there was no relationship between HHT genotype or control origin for success of establishment, time to first colony, or proliferation. We confirmed BOECs retained endothelial protein (CD31+, CD44+, CD105+, CD90−) and marker gene signatures (supplemental Figure 3; supplemental Table 1).
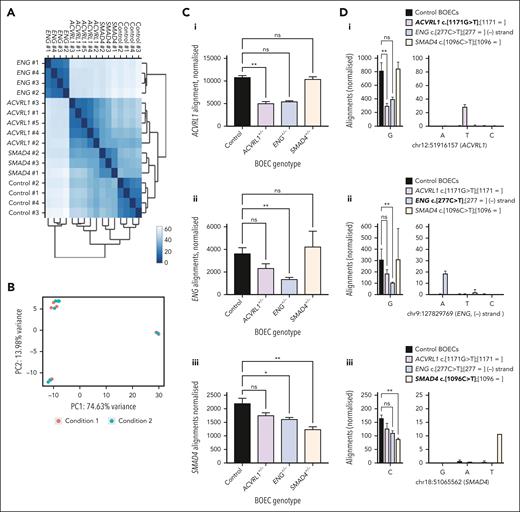
BOECs were used to derive libraries for RNA sequencing. Reads were of consistently high quality (supplemental Figure 4). Quantitative validations were performed for 48 genes that we had tested by both RNA sequencing and scqRT-PCR (supplemental Figure 3C). Overall, 59% of the variability in mean control BOEC RNA sequencing alignments normalized to total library count was accounted for by scqRT-PCR ranking expression (P < .0001; supplemental Figure 3C). In unsupervised Euclidean distance (Figure 2A) and principal component analyses (Figure 2B) of BOECs at steady state, 3 to 4 clusters were distinguished, and unblinding identified these corresponded to donor status. Unexpectedly, ACVRL1+/PTC/SMAD4+/PTC BOECs were more distinct to ENG+/PTC BOECs than to control BOECs (Figure 2A).
Steady state RNA sequencing in control and HHT BOECs. (A) Unsupervised Euclidean distance analyses for the 16 BOEC RNA sequencing data sets. When unblinded, donor replicates were seen to have clustered together. (B) Unsupervised principal component analysis of the 16 BOEC RNA sequencing data sets based on the distance matrix in which samples were projected to a 2-dimensional plane spanned by their first 2 principal components. Colored dots distinguish an experimental covariate (10 ng/mL BMP9 for 1 hour), which was discernible in control BOECs (top left) but not in the HHT clusters (ENG+/PTC and ACVRL1+/PTC/SMAD4+/PTC BOECs). (C-F) Genotype-specific data colored as for other figures (ACVRL1+/− pink/purple; ENG+/− blue; SMAD4+/− orange). (C) Normalized alignments to HHT gene Ensembl identifiers (i) ACVRL1 (ENSG00000139567), (ii) ENG (ENSG00000106991), and (iii) SMAD4 (ENSG00000141646) in control and HHT BOECs. Data from all samples per donor, mean and standard deviation shown, ∗∗P < .005 and ∗P < .05, by Dunn's test after Kruskal-Wallis test. (D) Alignments to nonsense alleles in ACVRL1, ENG, and SMAD4 nucleotides in GRCh38/hg3870 corresponding to donor heterozygous pathogenic variants. Left: wild-type allele at (i) chr12:51,916,158, (ii) chr9:127,829,769, and (iii) chr18:51065563. Right: alternate alleles at genomic position at 10× scale. Color-code key uses recommended75 nomenclature to describe both alleles per HHT donor: the relevant nonsense donor allele is indicated in bold. Mean and standard deviation displayed, pairwise P values (∗∗P < .005) calculated by Dunn's test after Kruskal-Wallis test; for overall P values P < .0001 [ACVRL1], P = .0002 [ENG], and P = .003 [SMAD4]. (E) Alignments to PTC allele in the HHT BOECs by genotype, color coded as in panels C and D. (i) Percentage of total expected if equal to wild-type allele alignment expected for heterozygotes. (ii) Percentage “loss” of total expected allele alignments. (F) Alignments to common single-nucleotide variants (SNVs) in ACVRL1 3′ UTR quantified by VarScan2.53 (i) GRCh38/hg3870 genomic positions of 6 common SNVs (V1-V6) in BOECs from donors heterozygous for ACVRL1 c.1171G>T, (p.Glu391X) or ENG c.277C>G, (p.Arg93X). Positions are illustrated by custom tracks uploaded to the UCSC Genome Browser.71 (ii) Variant: wild-type ratios in ACVRL1+/PTC and wild-type ACVRL1 (ACVRL1+/+; ENG+/PTC) BOECs. The higher ratios for V1 and V2 in ACVRL1+/PTC BOECs can be attributed to presence in cis with wild-type c.1171G, loss of the c.1171T in-frame transcripts generating the PTC, and presence of the SNV in all (V1), or only longer (V2) ACVRL1 3′ UTR transcripts (see also supplemental Figure 7).
Steady state RNA sequencing in control and HHT BOECs. (A) Unsupervised Euclidean distance analyses for the 16 BOEC RNA sequencing data sets. When unblinded, donor replicates were seen to have clustered together. (B) Unsupervised principal component analysis of the 16 BOEC RNA sequencing data sets based on the distance matrix in which samples were projected to a 2-dimensional plane spanned by their first 2 principal components. Colored dots distinguish an experimental covariate (10 ng/mL BMP9 for 1 hour), which was discernible in control BOECs (top left) but not in the HHT clusters (ENG+/PTC and ACVRL1+/PTC/SMAD4+/PTC BOECs). (C-F) Genotype-specific data colored as for other figures (ACVRL1+/− pink/purple; ENG+/− blue; SMAD4+/− orange). (C) Normalized alignments to HHT gene Ensembl identifiers (i) ACVRL1 (ENSG00000139567), (ii) ENG (ENSG00000106991), and (iii) SMAD4 (ENSG00000141646) in control and HHT BOECs. Data from all samples per donor, mean and standard deviation shown, ∗∗P < .005 and ∗P < .05, by Dunn's test after Kruskal-Wallis test. (D) Alignments to nonsense alleles in ACVRL1, ENG, and SMAD4 nucleotides in GRCh38/hg3870 corresponding to donor heterozygous pathogenic variants. Left: wild-type allele at (i) chr12:51,916,158, (ii) chr9:127,829,769, and (iii) chr18:51065563. Right: alternate alleles at genomic position at 10× scale. Color-code key uses recommended75 nomenclature to describe both alleles per HHT donor: the relevant nonsense donor allele is indicated in bold. Mean and standard deviation displayed, pairwise P values (∗∗P < .005) calculated by Dunn's test after Kruskal-Wallis test; for overall P values P < .0001 [ACVRL1], P = .0002 [ENG], and P = .003 [SMAD4]. (E) Alignments to PTC allele in the HHT BOECs by genotype, color coded as in panels C and D. (i) Percentage of total expected if equal to wild-type allele alignment expected for heterozygotes. (ii) Percentage “loss” of total expected allele alignments. (F) Alignments to common single-nucleotide variants (SNVs) in ACVRL1 3′ UTR quantified by VarScan2.53 (i) GRCh38/hg3870 genomic positions of 6 common SNVs (V1-V6) in BOECs from donors heterozygous for ACVRL1 c.1171G>T, (p.Glu391X) or ENG c.277C>G, (p.Arg93X). Positions are illustrated by custom tracks uploaded to the UCSC Genome Browser.71 (ii) Variant: wild-type ratios in ACVRL1+/PTC and wild-type ACVRL1 (ACVRL1+/+; ENG+/PTC) BOECs. The higher ratios for V1 and V2 in ACVRL1+/PTC BOECs can be attributed to presence in cis with wild-type c.1171G, loss of the c.1171T in-frame transcripts generating the PTC, and presence of the SNV in all (V1), or only longer (V2) ACVRL1 3′ UTR transcripts (see also supplemental Figure 7).
Endothelial cells maintain original HHT genotypes
To confirm that BOECs maintained their HHT genotypes, we examined alignments to each gene and nonsense substitution genomic site, blinded to donor. We expected to see reduced alignments to the nonsense allele gene because of nonsense-mediated decay, and this was observed: overall alignments to the gene harboring the mutant PTC allele were reduced (Figure 2Ci-iii).
As shown in Figure 2Di-iii, alignments were specifically reduced to the wild-type nucleotide substituted in the mutant PTC allele, with alignments to the PTC-generating nucleotide detected at lower and variable levels: of the 16 HHT and control BOECs, 12 demonstrated significant mismatches to GRCh38/hg3870 at chr12:51,916,158, chr9:127,829,769, or chr18:51,065,563 corresponding to BOEC donor genotypes ACVRL1 c.1171G>T, p.(Glu391X), ENG c.277C>T, p.(Arg93X), and SMAD4 c.1096C>T, p.(Gln368X; Figure 2D; supplemental Figure 5). In all cases, HHT nonsense allele alignments were within the expected BOECs, and aligned to spliced exons (almost all alignments sharply defined the exon–intron boundaries; supplemental Figure 6). All ratios were skewed from the ratio of 1:1 expected for germ line heterozygous alleles with a mean wild-type:nonsense allele ratio of 9:1 (range, 5:1-13:1; Figure 2E).
To provide separate evidence that PTC-containing transcripts were persisting at low levels, we performed allele-specific expression analyses using VarScan253 which had detected common variants in the ACVRL1 3′ UTR that, by chance, were present in 2 of the HHT donors. All 4 ACVRL1 3′ UTR heterozygous variants in ENG+/PTC BOECs (ie, ACVRL1+/+) displayed the expected 1:1 ratios (Figure 2Fi). However, the 2 ACVRL1 3′ UTR variants in the ACVRL1+/PTC BOECs were present in a greater proportion of transcripts (Figure 2Fii), consistent with being in cis (on the same RNA molecule) as the wild-type allele, with the PTC allele/transcript at a reduced level. This also demonstrated that a proportion of ACVRL1 PTC-containing transcripts persisted to the 3′ UTR, which would potentially permit in-frame protein translation.
Pathway analyses for differentially expressed genes
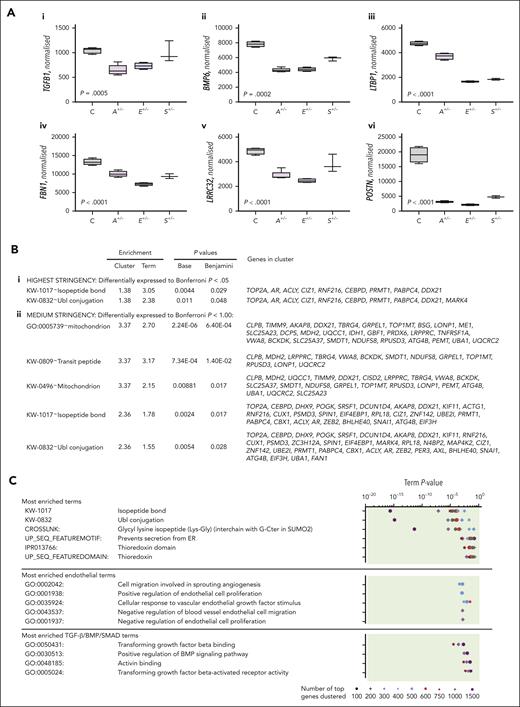
Genes encoding proteins in HHT causal pathways were differentially expressed in HHT+/PTC BOECs. These included disease-causing genes (ACVRL1, ENG, and SMAD4; Figure 2C); superfamily ligands (TGFB1 and BMP6; Figure 3Ai-ii), and extracellular matrix proteins involved in TGF-β/BMP ligand activation (Figure 3Aiii-vi). To explore differences beyond these already known, disease pathway-specific signals, unbiased Gene Ontology clustering63-65 was performed at 3 levels of stringency, with and without GINI gene normalization (supplemental Figure 8). For the highest stringency, we restricted analyses to the 30 genes that were differentially expressed between HHT and control BOECs to Bonferroni P < .05 from the 5013 meeting CV10 (supplemental Table 3A); at medium stringency, the 161 genes that met Bonferroni P < 1.00 and CV10 (supplemental Table 3B); and at the lowest stringency level, the top 100, 200, 300, 400, 500, 600, 750, 1000, or 1500 differentially expressed genes, normalized to total read counts per library (supplemental Table 4).
In pathway analyses across all 3 stringencies, a single significant cluster consistently emerged. This contained 2 terms, “isopeptide-bond” and “ubiquitin-like conjugation.” It was the only term at highest stringency (Figure 3Bi), 1 of 2 significant terms at medium stringency (Figure 3Bii), and also emerged repeatedly in lower stringency analyses when no significant clusters emerged from 10 sets of 1000 randomly selected genes from the same BOEC data sets (supplemental Figure 9). Notably, this cluster’s terms were not specifically related to endothelial function or BMP/TGF-β signaling predicted by the loss-of-function alleles (Figure 3C) but reflected overlapping biology of ubiquitin and ubiquitin-like peptides binding to protein substrates via isopeptide bonds to target for lysosomal degradation. The data were similar between different BOECs (Figure 3D), and consistent with differences in posttranslational protein processes between the PTC (HHT) and non-PTC (control) data sets.
Gene Ontology clustering of genes differentially expressed between HHT and control BOECs. (A) Expression of genes encoding TGF-β/BMP ligands ([i] TGF-β1, [ii] BMP6), and extracellular matrix BMP/TGF-β sequestration and activation proteins ([iii] LTBP1, [iv] FBN1, [v] LRRC32, and [vi] POSTN encoding latent TGFβ-binding protein 1, fibrillin 1, leucine-rich repeat containing 32, and periostin, respectively). Expression was DeSeq2 normalized59,60 using low–GINI coefficient genes61,62 that displayed least variability in BOECs (supplemental Table 2; supplemental Figure 8), although these genes were also differentially expressed when normalized only to total reads per library. Box plots show interquartile range and error bars (minimum to maximum). P values were calculated by Kruskal-Wallis test. Control (C) black, ACVRL1+/− (A+/−) is highlighted purple; ENG+/− (E+/−) blue; SMAD4+/− (S+/−) orange. (B) All significant terms enriched by clustering genes differentially expressed to (i) Bonferroni P < .05 (n = 30); and (ii) Bonferroni P < 1.00 (n = 161). (C) Specific terms enriched by clustering the most differentially expressed genes in each data set examining each category from top 100 to top 1500 separately. The lowest P value for each cluster is displayed. Note consistency with the most enriched terms displayed in panel B, and for further detail, see supplemental Figure 9. (D) Comparison of expression for each of the 9 “Bonferroni P < .05” genes that clustered to both isopeptide-bond and ubiquitin-like conjugation in panel B, by BOEC genotype color-coded as in panel A. Mean, standard deviation, and Kruskal-Wallis P values displayed; posttest pairwise P values by Dunn's test: ∗P < .05, ∗∗P < .01, and ∗∗∗P < .005; ns, not significant at P ≥ .05.
Gene Ontology clustering of genes differentially expressed between HHT and control BOECs. (A) Expression of genes encoding TGF-β/BMP ligands ([i] TGF-β1, [ii] BMP6), and extracellular matrix BMP/TGF-β sequestration and activation proteins ([iii] LTBP1, [iv] FBN1, [v] LRRC32, and [vi] POSTN encoding latent TGFβ-binding protein 1, fibrillin 1, leucine-rich repeat containing 32, and periostin, respectively). Expression was DeSeq2 normalized59,60 using low–GINI coefficient genes61,62 that displayed least variability in BOECs (supplemental Table 2; supplemental Figure 8), although these genes were also differentially expressed when normalized only to total reads per library. Box plots show interquartile range and error bars (minimum to maximum). P values were calculated by Kruskal-Wallis test. Control (C) black, ACVRL1+/− (A+/−) is highlighted purple; ENG+/− (E+/−) blue; SMAD4+/− (S+/−) orange. (B) All significant terms enriched by clustering genes differentially expressed to (i) Bonferroni P < .05 (n = 30); and (ii) Bonferroni P < 1.00 (n = 161). (C) Specific terms enriched by clustering the most differentially expressed genes in each data set examining each category from top 100 to top 1500 separately. The lowest P value for each cluster is displayed. Note consistency with the most enriched terms displayed in panel B, and for further detail, see supplemental Figure 9. (D) Comparison of expression for each of the 9 “Bonferroni P < .05” genes that clustered to both isopeptide-bond and ubiquitin-like conjugation in panel B, by BOEC genotype color-coded as in panel A. Mean, standard deviation, and Kruskal-Wallis P values displayed; posttest pairwise P values by Dunn's test: ∗P < .05, ∗∗P < .01, and ∗∗∗P < .005; ns, not significant at P ≥ .05.
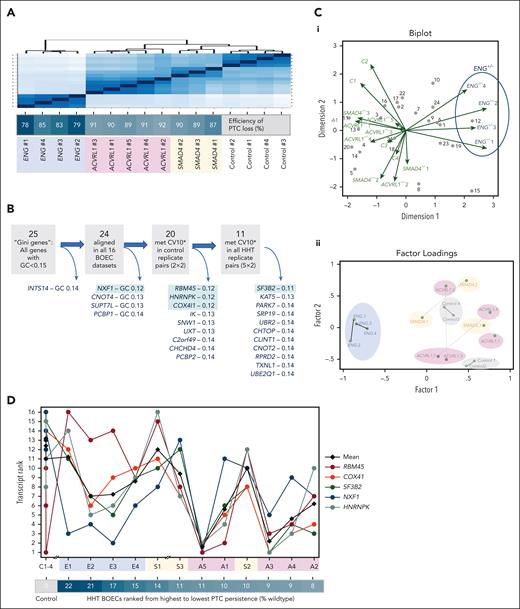
Unsupervised machine learning discriminates individual BOEC cultures by PTC persistence
The ENG+/PTC BOECs, already shown to resemble other HHT BOECs less than controls (Figures 2A and 4A), had least efficient degradation of PTC transcripts (Figure 2E). Surprisingly, the 4 cultures of ENG+/− BOECs were also demarcated by unsupervised multivariate analyses of the near-invariant GINI “housekeeper” genes, ranked by relative expression across the 16 BOEC cultures (Figure 4B-C). Because GINI housekeeper genes were similarly ranked across 24 donor–treatment combinations in peripheral blood mononuclear cells derived from patients with HHT without PTCs,76 we explored whether interculture variability may relate to persistence of PTC transcripts.
PTC sequence and endothelial contexts. As for other figures, ACVRL1+/− is highlighted in pink/purple; ENG+/− in blue, and SMAD4+/− in orange. (A) Unsupervised Euclidean distance analyses for the 16 BOEC RNA sequencing data sets: note efficiency of PTC loss was not associated with the differences identified by the initial global expression profiles. (B) BOEC expression of the 25 lowest Gini coefficient genes61,62 (GC < 0.15) with GC < 0.13 genes highlighted in green (details in supplemental Table 2; supplemental Figure 8). (C) Unsupervised analyses of the 24 BOEC-expressed GINI genes ranked in 16 BOEC cultures. (i) Multivariate analysis of genes (observations, gray dots) across the BOECs (variables, green arrows). Note demarcation of the 4 ENG+/PTC BOEC cultures. (ii) Principal factor analysis on 16 BOEC data sets using ranks of the 24 GINI genes. The 15 retained factors and 120 evaluated parameters again distinguished the ENG+/PTC BOEC cultures (blue) from ACVRL1+/PTC (pink); SMAD4+/PTC (orange); and control BOECs (gray). (D) Respective ranks of the 5 lowest GC genes (highlighted green in panel B) across BOEC cultures ranked by % PTC persistence and genotype (C: control, E: ENG+/PTC, S: SMAD4+/PTC, A: ACVRL1+/PTC). Note switch between 14% and 11% persistence for NXF1 (blue) and RBM45 (brown). Two-way analysis of variance (ANOVA) data are presented in supplemental Table 7.
PTC sequence and endothelial contexts. As for other figures, ACVRL1+/− is highlighted in pink/purple; ENG+/− in blue, and SMAD4+/− in orange. (A) Unsupervised Euclidean distance analyses for the 16 BOEC RNA sequencing data sets: note efficiency of PTC loss was not associated with the differences identified by the initial global expression profiles. (B) BOEC expression of the 25 lowest Gini coefficient genes61,62 (GC < 0.15) with GC < 0.13 genes highlighted in green (details in supplemental Table 2; supplemental Figure 8). (C) Unsupervised analyses of the 24 BOEC-expressed GINI genes ranked in 16 BOEC cultures. (i) Multivariate analysis of genes (observations, gray dots) across the BOECs (variables, green arrows). Note demarcation of the 4 ENG+/PTC BOEC cultures. (ii) Principal factor analysis on 16 BOEC data sets using ranks of the 24 GINI genes. The 15 retained factors and 120 evaluated parameters again distinguished the ENG+/PTC BOEC cultures (blue) from ACVRL1+/PTC (pink); SMAD4+/PTC (orange); and control BOECs (gray). (D) Respective ranks of the 5 lowest GC genes (highlighted green in panel B) across BOEC cultures ranked by % PTC persistence and genotype (C: control, E: ENG+/PTC, S: SMAD4+/PTC, A: ACVRL1+/PTC). Note switch between 14% and 11% persistence for NXF1 (blue) and RBM45 (brown). Two-way analysis of variance (ANOVA) data are presented in supplemental Table 7.
As shown in Figure 4D, the GINI gene rankings changed across the PTC BOECs. In particular, for the least variant (GC 0.11-0.12) genes, the respective rankings of RBM45 and NXF1 were switched between BOECs with ≥13% and ≤11% PTC persistence (Figure 4D). These “PTC persistence” categories explained 17% of the variance in GINI gene rankings (P = .0007), and a further 21% through the interaction term with gene (P = .005; supplemental Table 7). In contrast, categorizing by genotype was not significant (supplemental Table 7). We concluded that variability in GINI genes was evident and related to PTC persistence.
Because the ISR66 inhibits protein translation necessary for NMD40-43 and cellular stress is one of the most robust regulators of NMD,66,77,78 PTC transcripts could be persisting more in cells that were already stressed. Furthermore, the ISR would predict the RMB45/NXF1 switch. This is because the ISR inhibits bulk messenger RNA (mRNA) nuclear export,79 including via RNA binding motif protein 45 (RBM45) that binds to mRNAs and forms reversible nuclear inclusions during stress,80,81 and mRNA dissociation from nuclear RNA export factor 1 (NXF1) required for bulk export of mRNAs.79,82 However, associations can be in either direction, and we next tested the possibility that PTC transcript persistence could be causally related to cellular stress.
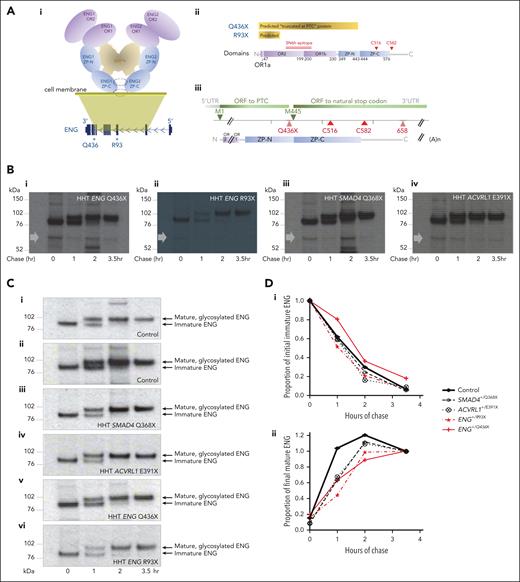
Pulse-chase examination of ENG protein in BOECs
We first tested mutant protein production in patient-derived BOECs, focusing on ENG, which had well-established mAbs (PD31, P4A4, and SN6h72,83) and highest PTC transcript persistence (Figure 2E). Although most HHT-causal ENG nonsense variants are too proximal (5′) for predicted proteins to contain both OR domains that span the mAb epitopes (Figure 5A), we were able to establish BOECs from a patient with HHT heterozygous for a rare nonsense substitution (ENG c.1306C>T) generating a PTC at Q436, at the end of the ZP-N domain. This predicted that the complete OR region (E26-C330) containing all SN6h epitope residues (A119-G230)72 would be present in a lower intensity, smaller band corresponding to a molecule truncated at the PTC approximately two-thirds (436 of 658 amino acids) through the protein (Figure 5A). However, pulse-chase patterns spanning the expected position were no different in ENG+/Q436X BOECs compared with other HHT BOECs (Figure 5B).
ENG protein expression in normal and HHT endothelial cells. (A) Schematic representations of ENG protein. (i) Domain organization of the ENG protein ectodomain in its BMP-binding homodimeric conformation, in relation to its genomic DNA origin, with OR domains highlighted in purple and ZP domains in blue. Q436 is distal to OR domains OR1 and OR2, but proximal to intermolecular dimerization cysteines C516 and C582 located within and after the ZP-C domain, respectively.72 For simplicity, the single-spanning C-terminal transmembrane domain of ENG (residues 587-611) is not shown. (ii) “Predicted” PTC-truncated ENG Q436X and R93X proteins in relation to the domains and SN6h epitope of wild-type ENG.72,83 (iii) ENG Q436X open reading frames from methionine 1 (M1) to PTC, and M445 to natural stop codon. (B) Pulse-chase experiments in which BOEC lysates were size fractionated on a reducing gel, and overexposed to optimize detection of minor, smaller bands. The approximate position of the Q436X protein that should be detectable by the 35S-labeled SN6h antibody is indicated by a gray arrow in BOECs heterozygous for (i) ENG Q436X, (ii) ENG R93X, (iii) SMAD4 Q368X, and (iv) ACVRL1 E391X. Note, no difference in patterns of sub–full length proteins in HHT BOECs, providing no evidence for a Q436X-specific truncated protein product at expected size based on truncation at the PTC (for full gel data see supplemental Figure 10). (C) Comparison of maturation patterns of full-length ENG protein after pulse-chase in BOECs heterozygous for (i-iv) ENG+/+ (wild-type) controls, and (v-vi) ENG+/PTC BOECs: (i) control A; (ii) control B; (iii) SMAD4 Q368X; (iv) ACVRL1 E391X, (v) exon 10 ENG Q436X, and (vi) exon 3 ENG R93X. (D) Densitometry analyses of the immunoblots in panel C. (i) Lower band (immature ENG) densitometry values across the 4 chase time points. By 2-way ANOVA, time accounted for 72.5% of variation (P < .0001), ENG vs non-ENG genotypes for 1.8% (P = .049), and the interaction term for 0.98% (P = .092). (ii) Upper band (mature ENG) densitometry values across the 4 chase time points. By 2-way ANOVA, time accounted for 64.3% of variation (P < .0001), ENG vs non-ENG genotypes for 1.4% (P = .54), and the interaction term for 3.5% (P = .26).
ENG protein expression in normal and HHT endothelial cells. (A) Schematic representations of ENG protein. (i) Domain organization of the ENG protein ectodomain in its BMP-binding homodimeric conformation, in relation to its genomic DNA origin, with OR domains highlighted in purple and ZP domains in blue. Q436 is distal to OR domains OR1 and OR2, but proximal to intermolecular dimerization cysteines C516 and C582 located within and after the ZP-C domain, respectively.72 For simplicity, the single-spanning C-terminal transmembrane domain of ENG (residues 587-611) is not shown. (ii) “Predicted” PTC-truncated ENG Q436X and R93X proteins in relation to the domains and SN6h epitope of wild-type ENG.72,83 (iii) ENG Q436X open reading frames from methionine 1 (M1) to PTC, and M445 to natural stop codon. (B) Pulse-chase experiments in which BOEC lysates were size fractionated on a reducing gel, and overexposed to optimize detection of minor, smaller bands. The approximate position of the Q436X protein that should be detectable by the 35S-labeled SN6h antibody is indicated by a gray arrow in BOECs heterozygous for (i) ENG Q436X, (ii) ENG R93X, (iii) SMAD4 Q368X, and (iv) ACVRL1 E391X. Note, no difference in patterns of sub–full length proteins in HHT BOECs, providing no evidence for a Q436X-specific truncated protein product at expected size based on truncation at the PTC (for full gel data see supplemental Figure 10). (C) Comparison of maturation patterns of full-length ENG protein after pulse-chase in BOECs heterozygous for (i-iv) ENG+/+ (wild-type) controls, and (v-vi) ENG+/PTC BOECs: (i) control A; (ii) control B; (iii) SMAD4 Q368X; (iv) ACVRL1 E391X, (v) exon 10 ENG Q436X, and (vi) exon 3 ENG R93X. (D) Densitometry analyses of the immunoblots in panel C. (i) Lower band (immature ENG) densitometry values across the 4 chase time points. By 2-way ANOVA, time accounted for 72.5% of variation (P < .0001), ENG vs non-ENG genotypes for 1.8% (P = .049), and the interaction term for 0.98% (P = .092). (ii) Upper band (mature ENG) densitometry values across the 4 chase time points. By 2-way ANOVA, time accounted for 64.3% of variation (P < .0001), ENG vs non-ENG genotypes for 1.4% (P = .54), and the interaction term for 3.5% (P = .26).
There were subtle differences in ENG protein processing in ENG+/Q436X BOECs. In control, SMAD4+/PTC, and ACVRL1+/PTC BOECs, newly synthesized ENG was predominantly immature, and the mature protein became the more pronounced species after 1 hour and maximal after 2 hours of chase (Figure 5Ci-iv). Similar patterns were evident in ENG+/R93X (Figure 5Cvi) BOECs. For ENG+/Q436X BOECs, immature ENG remained the more pronounced species at 1 hour, with traces still present at 2 hour (Figure 5Cv), while densitometry values for mature ENG peaked later (Figure 5Cv,D).
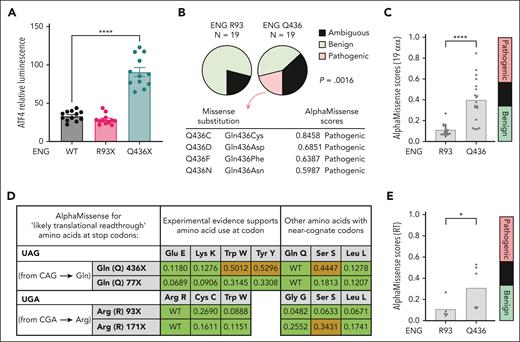
ENG Q436X but not ENG R93X induces the ISR
Next, we tested the ability of ENG mutants to induce the ISR using ATF4 expression as an ISR read out.66,67 After transient transfection of ATF4::nanoLuc reporter cells, expression of ENG Q436X but not ENG R93X directly induced ATF4 (Figure 6A).
ENG 436X and ENG 93X comparisons. (A) ATF4::nanoLuc reporter system to detect induction of the ISR/eIF2α activation, using ATF4 expression as an ISR readout.67P value calculated by Dunn's test after Kruskal-Wallis test. Shown are results from 3 biological replicate transfections on separate days, each with 4 technical replicates. ∗∗∗∗P < .001. (B-E) Analysis of benign (green), ambiguous (blue in panels B,C,E; brown in panel D) and pathogenic (red) missense substitution predictions by AlphaMissense. (B) Comparison of benign/ambiguous/pathogenic predictions for all 19 alternative amino acids at ENG R93 and ENG R436. Identity and metrics for all predicted pathogenic missense substitutions are shown. P value calculated by Fisher's exact test. (C) Distribution of AlphaMissense metrics for all 19 possible substitutions at ENG R93 and Q436, ∗∗∗∗P < .001 calculated by Mann-Whitney U test. (D) AlphaMissense predictions for the amino acids most likely to be substituted if translational (ribosomal) readthrough occurs at R93X and Q436X. Displayed are the stop codon sequences, the specific amino acids encoded by near-cognate codons distinguishing those with experimental evidence, and the AlphaMissense pathogenicity scores and predictions. (E) Comparison of AlphaMissense metrics for the amino acids most likely to be substituted if translational (ribosomal) readthrough occurs at ENG R93 and Q436; ∗P < .05 calculated by Mann-Whitney U test. RLU, relative luminescence units.
ENG 436X and ENG 93X comparisons. (A) ATF4::nanoLuc reporter system to detect induction of the ISR/eIF2α activation, using ATF4 expression as an ISR readout.67P value calculated by Dunn's test after Kruskal-Wallis test. Shown are results from 3 biological replicate transfections on separate days, each with 4 technical replicates. ∗∗∗∗P < .001. (B-E) Analysis of benign (green), ambiguous (blue in panels B,C,E; brown in panel D) and pathogenic (red) missense substitution predictions by AlphaMissense. (B) Comparison of benign/ambiguous/pathogenic predictions for all 19 alternative amino acids at ENG R93 and ENG R436. Identity and metrics for all predicted pathogenic missense substitutions are shown. P value calculated by Fisher's exact test. (C) Distribution of AlphaMissense metrics for all 19 possible substitutions at ENG R93 and Q436, ∗∗∗∗P < .001 calculated by Mann-Whitney U test. (D) AlphaMissense predictions for the amino acids most likely to be substituted if translational (ribosomal) readthrough occurs at R93X and Q436X. Displayed are the stop codon sequences, the specific amino acids encoded by near-cognate codons distinguishing those with experimental evidence, and the AlphaMissense pathogenicity scores and predictions. (E) Comparison of AlphaMissense metrics for the amino acids most likely to be substituted if translational (ribosomal) readthrough occurs at ENG R93 and Q436; ∗P < .05 calculated by Mann-Whitney U test. RLU, relative luminescence units.
The most likely mechanism would be through poorly folded mutant proteins causing a proteostasis load. Because we had not identified Q436X PTC-truncated protein by SN6h labeling/pulse chase in native BOECs, we considered how aberrant proteins that would escape SN6h detection could be generated, by ribosomal re-initiation42,84-86 or by ribosomal readthrough42,84-86 (supplemental Table 8). We reasoned that because AlphaFold can accurately model the domains of ENG harboring these residues (supplemental Figure 11), we could take advantage of publicly available AlphaMissense74 predictions to computationally test whether full-length ENG readthrough proteins with a single missense substitution at the respective stop codon might generate proteostatic stress. Four potential amino acid substitutions were predicted as pathogenic at Q436, with no pathogenic predictions for R93 (Figure 6B) which had lower AlphaMissense scores across all 19 alternative amino acids (Figure 6C). However, not all amino acids are substituted in readthrough, with nonrandom incorporation reflecting similarity to the cognate codon.42,84-86 For Q436X (TAG stop codon), 3 likely substitutions including 2 supported by experimental evidence86 had a higher “ambiguous” AlphaMissense score (Figure 6D), and restricting to near-cognate codons, AlphaMissense scores were higher than for R93X (Figure 6E). These data indicate that if natural readthrough occurred, any new “missense” substitution would likely be tolerated in R93X, whereas at Q436X it could cause aberrant protein folding/proteostatic stress consistent with Figure 6A.
PTC readthrough and allele frequency considerations
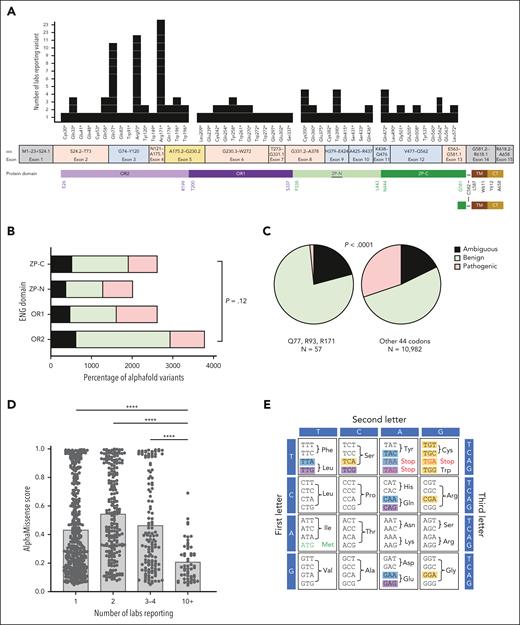
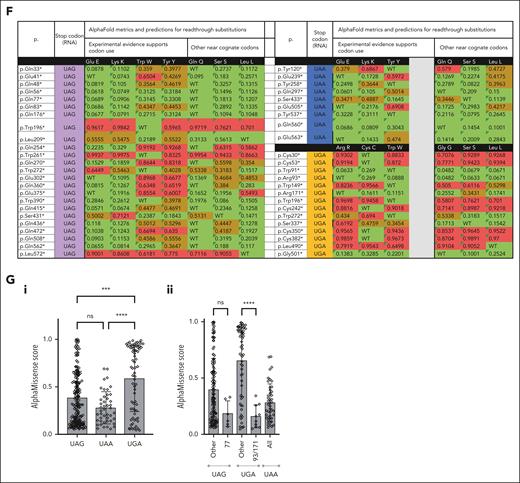
NMD transcripts are known to persist at higher levels in “less rare” PTCs.40 We recently reported that ENG R93X is the most common HHT causal variant in our UK-based HHT series,68 but in the general population, Arg93X and Q436X were each reported only once in GnomAD 4.0.0.69 We therefore examined a larger ENG PTC-enriched population, an HHT mutation database containing 47 different HHT-causal ENG nonsense variants encoded by exons 2 to 13 of the 15 exon gene (C30X-L572X; Figure 7A). R93X was the second most commonly reported ENG nonsense variant, reported by 11 separate laboratories worldwide, whereas Q436X was reported only twice. In total, 44 (93.6%) ENG nonsense variants were reported by fewer than 4 laboratories. The more common variants (Q77X, R93X, and R171X) all map to the proximal ENG OR2 domain (Figure 7A). Benign, ambiguous, and pathogenic AlphaMissense predictions did not differ overall for codons in OR2 compared with the other 3 ENG domains (Figure 7B). Categorizing by nonsense variant rarity, however, positions Q77, R93, and R171 had fewer pathogenic predictions than the remaining 44, across all amino acids (Figure 7C-D), or restricting to near-cognate codons (Figure 7E-G). Thus, although there was no clear relationship to length of any PTC-truncated protein, the 3 nonsense variants most commonly reported in HHT were at sites at which naturally occurring readthrough would be best tolerated, partially correcting loss of function.
Broader pathogenic ENG PTC variant considerations. For color codes, ENG protein domains (A) are colored as in Figure 5 and AlphaMissense74 predictions as benign (green), ambiguous (black in panels B-C; and brown in panel F), and pathogenic (red). For stop codon origins in panels E-F, these are colored purple (TAG/UAG); blue (TAA/UAA), or orange (TGA/UGA). (A) Distribution of the 2018 HHT mutation database–listed nonsense variants. Note that all were located between exons 2 and 13 of the 15 exon gene; variants were similarly spread between codons in the 4 protein domains, but the 3 most common variants (Q77X, R93X, and R171X) were all in the OR2 domain (Figure 5A; supplemental Figure 11). Amino acids flanking the domain junctions, and the PTC-distal site of intermolecular dimerization at C582 are also shown. (B) Comparison of AlphaMissense predictions for all 19 alternate amino acids at each nonsense codon site, categorized by protein domain. P value calculated by Fisher's exact test. (C) Comparison of AlphaMissense metrics for all 19 alternate amino acids at each nonsense codon site, categorized by most common (Q77X, R93X, and R171X) vs other (n = 44) HHT causal nonsense variants. P value calculated by Mann-Whitney U test. Note, paucity of pathogenic predictions for Q77X, R93X, and R171X. (D) Distribution of AlphaMissense pathogenicity scores categorized by common (Q77X, R93X, and R171X) vs other (n = 44) stop codons. ∗∗∗∗P < .0001 calculated by Dunn's test after Kruskal-Wallis test. (E) Genetic code indicating the 12 nonsense source codons in the HHT series, and the 3 resultant nonsense mutant codons. For further detail see supplemental Table 9A. (F) AlphaMissense predictions for the amino acids most likely to be substituted if translational (ribosomal) readthrough occurs, by stop codon type. For further detail see supplemental Table 8B. (G) AlphaMissense predictions for the amino acids most likely to be substituted if translational (ribosomal) readthrough occurs, by stop codon type. (i) All stop codons; and (ii) subcategorizing by more common variants (Q77X, R93X, and R171X). ∗∗∗∗P < .0001 and ∗∗∗P < .001 calculated by Dunn's test after Kruskal-Wallis test.
Broader pathogenic ENG PTC variant considerations. For color codes, ENG protein domains (A) are colored as in Figure 5 and AlphaMissense74 predictions as benign (green), ambiguous (black in panels B-C; and brown in panel F), and pathogenic (red). For stop codon origins in panels E-F, these are colored purple (TAG/UAG); blue (TAA/UAA), or orange (TGA/UGA). (A) Distribution of the 2018 HHT mutation database–listed nonsense variants. Note that all were located between exons 2 and 13 of the 15 exon gene; variants were similarly spread between codons in the 4 protein domains, but the 3 most common variants (Q77X, R93X, and R171X) were all in the OR2 domain (Figure 5A; supplemental Figure 11). Amino acids flanking the domain junctions, and the PTC-distal site of intermolecular dimerization at C582 are also shown. (B) Comparison of AlphaMissense predictions for all 19 alternate amino acids at each nonsense codon site, categorized by protein domain. P value calculated by Fisher's exact test. (C) Comparison of AlphaMissense metrics for all 19 alternate amino acids at each nonsense codon site, categorized by most common (Q77X, R93X, and R171X) vs other (n = 44) HHT causal nonsense variants. P value calculated by Mann-Whitney U test. Note, paucity of pathogenic predictions for Q77X, R93X, and R171X. (D) Distribution of AlphaMissense pathogenicity scores categorized by common (Q77X, R93X, and R171X) vs other (n = 44) stop codons. ∗∗∗∗P < .0001 calculated by Dunn's test after Kruskal-Wallis test. (E) Genetic code indicating the 12 nonsense source codons in the HHT series, and the 3 resultant nonsense mutant codons. For further detail see supplemental Table 9A. (F) AlphaMissense predictions for the amino acids most likely to be substituted if translational (ribosomal) readthrough occurs, by stop codon type. For further detail see supplemental Table 8B. (G) AlphaMissense predictions for the amino acids most likely to be substituted if translational (ribosomal) readthrough occurs, by stop codon type. (i) All stop codons; and (ii) subcategorizing by more common variants (Q77X, R93X, and R171X). ∗∗∗∗P < .0001 and ∗∗∗P < .001 calculated by Dunn's test after Kruskal-Wallis test.
Discussion
Frameshift and nonsense mutations are common causes of genetic disease, but why some transcripts escape NMD and persist at variable levels across tissues, and whether this is broadly relevant to human health, was not known. Here, we demonstrate by RNA sequencing that replicate endothelial cultures from the same donor retained differing proportions of nonsense PTC transcripts, could be discriminated in unsupervised hierarchical clustering, and that a cellular stress signature was identified in cells with higher PTC transcript expression. By pulse chase, we found no evidence for ENG Q436-truncated protein that should have been detected by the mAb used. Expression of ENG Q436X directly induced the ISR, but this was not seen for ENG R93X for which mAb-invisible PTC-truncated protein would be shorter, and missense substitutions resulting from ribosomal readthrough are predicted to be benign. Hemorrhage phenotypes in 3 different HHT populations suggested clinical relevance for these “generic” cellular phenotypes from PTC-generating mutations, beyond conventional pathway-specific perturbations that have been the focus of models for human genetic disease.
Study strengths include the integration of recent developments in RNA biology with clinical medicine, and design elements that focused on variant examination in endogenous cells from carefully selected donors to examine the least complex PTC variants (frameshift variants would introduce a run of alternate amino acids before the PTC).38 Confidence in discovery analyses was enhanced by scqRT-PCR validations; restricting analyses to genes with low intrareplicate variability; focusing on findings robust to different analytic methods; using exacting pulse-chase characterization of primary, nontransformed endothelial cells in as close to the natural state as possible; and testing confidence of structural predictions by AlphaFold before examining AlphaMissense metrics. The main weakness is the relatively small number of BOEC data sets examined, although this has been a more rigorous evaluation across all 3 major HHT genotypes in a way that, to our knowledge, has not been previously performed because of the rarity of the SMAD4+/PTC genotype in HHT.2,76 Additionally, this study does not distinguish processes generically associated with a PTC, from processes that are specific to HHT cells, and we cannot exclude changes in some way being augmented for HHT/ENG PTCs.
Our findings support previous science. Other diseases show differences between PTC-generating and non–PTC-generating variants, for example, Duchenne and Becker muscular dystrophies are both caused by DMD variants with the milder (non-PTC) Becker phenotype exploited in therapeutic strategies for the more severe (PTC) Duchenne muscular dystrophy.87 Genes differentially expressed in HHT/PTC BOECs clustered to ubiquitin-like pathways, and recent experimental data identified a ubiquitin-based pathway for nascent protein degradation from PTC-containing mRNAs.88 Because ribosomal readthrough would allow PTC transcripts to escape NMD,39-43,84-86 the robust evidence for more efficient NMD in less-rare (allele frequency >10−6) PTC variants40 would be supported by our data, if better tolerance of missense substitutions confers even a modest89 cellular survival advantage. Similarly, our data suggest testable explanations for why NMD efficiency rates differ across tissues,39,40 without systematic effects suitable for predictive models40—varying levels of stress in different tissues in different individuals at different time points.
For HHT, a new model is proposed in which some causal DNA variants do not “simply” result in loss-of-function but may also operate independently as a source or amplifier of cellular stress/injury. Mechanistic paradigms already lean toward concepts of second or third hits in addition to a single null allele,3-5,19,20 in keeping with evolving evidence for somatic mutation rates90,91 that may92 or may not93 result in clonal expansion if conferring advantages to the cell,92,94 and elegant studies confirming that injury, inflammatory and/or angiogenic stimuli can induce arteriovenous malformations in various models.3,4,12-15,20,95 Our data incorporate new stress considerations, whether by a PTC driving stress (as for Q436X), or increases in PTC transcript prevalence as a result of stress, according to protein location and precise stop codon sequence. Mutant proteins from most ENG PTCs are predicted to be more challenging for protein degradation pathways. Where PTC transcripts persist, our data are more consistent with ribosomal readthrough and incorporation of missense substitutions tolerated to differing degrees, than generation of peptides truncated at the PTC. In patient cells, where aberrant protein expression would be low, governed not only by low level RNA transcripts but also by usually rapid endoplasmic reticulum–associated degradation (ERAD)96 and/or lysosomal97 degradation, further studies will be required to establish whether and when levels are negligible or not negligible, and whether this differs between missense variants for which cellular processes can adapt to chronic proteotoxic loads,98,99 and PTC variants for which levels are more fluctuant.
There are 2 immediate pathways to translational impact. Our initial considerations were for the HHT vascular pathologies, to support genetic molecular subtype stratifications to augment the power of HHT clinical trials and likelihood that when a treatment is beneficial to a group or subgroup of patients, that benefit will be identified. For example, the clinical data point to nasal hemorrhage being more severe in patients with HHT ENG+/PTC genotypes, and more amenable to reduction of nasal Staphylococcus aureus load, particularly in an ointment specifically developed to suit the nasal mucosa. The data are of further relevance when considering the potential of exogenous ALK1 depletion to treat atherosclerotic pathology in the general population68,100 in which strategies that have any potential to generate mutant ALK1 protein would be discouraged.
In summary, we provide evidence from HHT populations and endothelial cells that support new genotypic classification systems and understanding of PTC-generating variants. Informed experiments that directly compare a larger number of PTCs/genes, other causes of haploinsufficiency, and ribosomal readthrough proteins in different stress states, will need to be the subject of future work. This may be especially relevant for cases in which the expected phenotypes impact cell growth and proliferation.
Acknowledgments
The authors thank the blood outgrowth endothelial cell donors for their participation in these studies.
The project received specific funding from The US Department of Defense Discovery Award W81XWH-16-1-0607 (M.A.A., C.L.S., Stopping the stops: a novel therapeutic approach for hemorrhage from vascular malformations); The National Institute for Health Research Imperial BRC Institute for Translational Medicine and Therapeutics (C.L.S., M.A.A.; Nonsense readthrough topical therapies for patients with inherited genetic diseases: proof of concept studies in hereditary haemorrhagic telangiectasia); Imperial College Healthcare National Health Service Trust (C.L.S., M.N.; Toward NHS Laboratory functional assays of genomic variants of uncertain significance, defining shared monocyte/target endothelial signatures in inherited vasculopathies); and The Averil Macdonald Memorial Fund and the D'Almeida Charitable Trust. L.J. was supported by the Knut and Alice Wallenberg Foundation (grant 2018.0042) and the Swedish Research Council (grant 2020-04936). MN was supported by the British Heart Foundation Center for Research Excellence at Imperial College London. M.A.A. was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute (grant R35HL140019). The study accessed data from the Genotype-Tissue Expression Project which was supported by the Common Fund of the Office of the Director of the National Institutes of Health; the data used for the analyses described in this manuscript were obtained from arteries and single endothelial cells, and extracted from the GTEx Portal on 19 April 2022.
The views expressed are those of the authors and not necessarily those of funders, the National Health Service, the National Institute for Health and Care Research, or the Department of Health and Social Care.
Authorship
Contribution: C.L.S. was responsible for study conceptualization; M.E.B.-H., D.P., A.B., J.Z., G.E., P.C.G., L.J., M.N., S.J.M., M.A.A., and C.L.S. were responsible for methodology; M.E.B.-H., D.P., K.J., A.B., J.Z., P.C.G., L.J., M.N., and C.L.S. were responsible for investigation; D.P., J.Z., P.C.G., L.J., M.N., and C.L.S. were responsible for visualization; M.A.A. and C.L.S. were responsible for funding acquisition; M.A.A. and C.L.S. were responsible for project administration; M.E.B.-H., D.P., M.N., S.J.M., M.A.A., and C.L.S. were responsible for supervision; C.L.S. was responsible for writing the original manuscript draft; M.E.B.-H., L.J., S.J.M., M.A.A., and C.L.S. were responsible for reviewing and editing the manuscript; M.E.B.-H., M.A.A., and C.L.S. devised the blood outgrowth endothelial cell (BOEC) strategy; M.E.B.-H., D.P., and M.A.A. performed BOEC cultures; D.P. prepared RNA, codesigned pulse-chase experiments, performed pulse-chase experiments, and generated the blots in Figure 5B-C; A.B. performed early RNA sequencing analyses, devised the CV10 approach, and contributed to single-cell quantitative reverse transcription polymerase chain reaction (scqRT-PCR) design; J.Z. performed ATF4 induction studies and generated Figure 6A; K.J. performed initial analysis of the clinical data set; I.S.M. devised the antibiotic ointment regime; P.C.G. and M.N. designed and performed scqRT-PCR and bulk flow cytometric analyses, and generated data for supplemental Table 1 and supplemental Figure 3A-B; L.J. generated AlphaFold predictions and analyzed ENG protein models (supplemental Figure 11); S.J.M. supervised J.Z. and G.E., and advised on the integrated stress response; C.L.S. reviewed and phenotyped patients, recruited patients, contributed to scqRT-PCR and pulse-chase experimental design, performed data analyses generating all other figures and tables; and all authors reviewed and approved the final manuscript.
Conflict-of-interest disclosure: The use of MEK1 inhibitors for treatment of HHT bleeding is the subject of a patent application by Imperial College London.
Correspondence: Claire L. Shovlin, National Heart and Lung Institute, Imperial Centre for Translational and Experimental Medicine, Imperial College London, Hammersmith Campus, Du Cane Rd, London W12 0NN, United Kingdom; email: c.shovlin@imperial.ac.uk; and Micheala A. Aldred, Division of Pulmonary, Critical Care, Sleep and Occupational Medicine, Department of Medicine, Indiana University School of Medicine, 980 W Walnut St, Indianapolis, IN 46202; email: maaldred@iu.edu.
References
Author notes
A.B., J.Z., and K.J. contributed equally to this study.
Nonsensitive data underlying this article are available at 10.5281/zenodo.5201823 and can be used under the Creative Commons Attribution license. Primary sequence data used in this research was collected subject to the participants’ informed consent. Access to these data will only be granted in line with that consent, subject to approval by the project ethics board and under a data sharing agreement. Blood outgrowth endothelial cells used in this research were collected subject to the informed consent of the participants. Access will only be granted in line with that consent, subject to approval by the project ethics board and under a material transfer agreement.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Steady state RNA sequencing in control and HHT BOECs. (A) Unsupervised Euclidean distance analyses for the 16 BOEC RNA sequencing data sets. When unblinded, donor replicates were seen to have clustered together. (B) Unsupervised principal component analysis of the 16 BOEC RNA sequencing data sets based on the distance matrix in which samples were projected to a 2-dimensional plane spanned by their first 2 principal components. Colored dots distinguish an experimental covariate (10 ng/mL BMP9 for 1 hour), which was discernible in control BOECs (top left) but not in the HHT clusters (ENG+/PTC and ACVRL1+/PTC/SMAD4+/PTC BOECs). (C-F) Genotype-specific data colored as for other figures (ACVRL1+/− pink/purple; ENG+/− blue; SMAD4+/− orange). (C) Normalized alignments to HHT gene Ensembl identifiers (i) ACVRL1 (ENSG00000139567), (ii) ENG (ENSG00000106991), and (iii) SMAD4 (ENSG00000141646) in control and HHT BOECs. Data from all samples per donor, mean and standard deviation shown, ∗∗P < .005 and ∗P < .05, by Dunn's test after Kruskal-Wallis test. (D) Alignments to nonsense alleles in ACVRL1, ENG, and SMAD4 nucleotides in GRCh38/hg3870 corresponding to donor heterozygous pathogenic variants. Left: wild-type allele at (i) chr12:51,916,158, (ii) chr9:127,829,769, and (iii) chr18:51065563. Right: alternate alleles at genomic position at 10× scale. Color-code key uses recommended75 nomenclature to describe both alleles per HHT donor: the relevant nonsense donor allele is indicated in bold. Mean and standard deviation displayed, pairwise P values (∗∗P < .005) calculated by Dunn's test after Kruskal-Wallis test; for overall P values P < .0001 [ACVRL1], P = .0002 [ENG], and P = .003 [SMAD4]. (E) Alignments to PTC allele in the HHT BOECs by genotype, color coded as in panels C and D. (i) Percentage of total expected if equal to wild-type allele alignment expected for heterozygotes. (ii) Percentage “loss” of total expected allele alignments. (F) Alignments to common single-nucleotide variants (SNVs) in ACVRL1 3′ UTR quantified by VarScan2.53 (i) GRCh38/hg3870 genomic positions of 6 common SNVs (V1-V6) in BOECs from donors heterozygous for ACVRL1 c.1171G>T, (p.Glu391X) or ENG c.277C>G, (p.Arg93X). Positions are illustrated by custom tracks uploaded to the UCSC Genome Browser.71 (ii) Variant: wild-type ratios in ACVRL1+/PTC and wild-type ACVRL1 (ACVRL1+/+; ENG+/PTC) BOECs. The higher ratios for V1 and V2 in ACVRL1+/PTC BOECs can be attributed to presence in cis with wild-type c.1171G, loss of the c.1171T in-frame transcripts generating the PTC, and presence of the SNV in all (V1), or only longer (V2) ACVRL1 3′ UTR transcripts (see also supplemental Figure 7).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/22/10.1182_blood.2023021777/2/m_blood_bld-2023-021777-gr2ef.jpeg?Expires=1769228332&Signature=MoISLFwN8nyB0RwcZvTlivI6QvdiqVYuyf60ToG61d-0sc8E5PD6zBAY1mz2H2NX1eqkIX8fiKmJD5j7UYabDm7g-jAMTcDiUFz7Bk7GlHThwgRJpTmaoGgT7DoIiuvGEPVQ0EbLigqxfJIvShtFqKasNsE87S5QMPRi2F7gwHf2PCqo1tqM5iVKuXzfo3zRJ7N86Q24l~4jBnxQp~i9sr84wkuHMHBtDF5uJGEamVyODoRynAMCMB01qSW3CB~xatZyOJN9KTwdWem53qRdj2bZVdbPL-c6KzhQqhrnwU8FTaqM5T729c9n1bUIW5UWPMzYfO5r6f0mP~GZb5mIrg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Gene Ontology clustering of genes differentially expressed between HHT and control BOECs. (A) Expression of genes encoding TGF-β/BMP ligands ([i] TGF-β1, [ii] BMP6), and extracellular matrix BMP/TGF-β sequestration and activation proteins ([iii] LTBP1, [iv] FBN1, [v] LRRC32, and [vi] POSTN encoding latent TGFβ-binding protein 1, fibrillin 1, leucine-rich repeat containing 32, and periostin, respectively). Expression was DeSeq2 normalized59,60 using low–GINI coefficient genes61,62 that displayed least variability in BOECs (supplemental Table 2; supplemental Figure 8), although these genes were also differentially expressed when normalized only to total reads per library. Box plots show interquartile range and error bars (minimum to maximum). P values were calculated by Kruskal-Wallis test. Control (C) black, ACVRL1+/− (A+/−) is highlighted purple; ENG+/− (E+/−) blue; SMAD4+/− (S+/−) orange. (B) All significant terms enriched by clustering genes differentially expressed to (i) Bonferroni P < .05 (n = 30); and (ii) Bonferroni P < 1.00 (n = 161). (C) Specific terms enriched by clustering the most differentially expressed genes in each data set examining each category from top 100 to top 1500 separately. The lowest P value for each cluster is displayed. Note consistency with the most enriched terms displayed in panel B, and for further detail, see supplemental Figure 9. (D) Comparison of expression for each of the 9 “Bonferroni P < .05” genes that clustered to both isopeptide-bond and ubiquitin-like conjugation in panel B, by BOEC genotype color-coded as in panel A. Mean, standard deviation, and Kruskal-Wallis P values displayed; posttest pairwise P values by Dunn's test: ∗P < .05, ∗∗P < .01, and ∗∗∗P < .005; ns, not significant at P ≥ .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/22/10.1182_blood.2023021777/2/m_blood_bld-2023-021777-gr3d.jpeg?Expires=1769228332&Signature=CgmsAGyWqVwnhZClwO1W0JjP54PMcBDXvcm5qJXk-9OoT0Fiu6bsdEWJNLwLzO6d29bewaqNkBCECC3NuvM-lM2o1ApXFtiJZol5wrFoyIaYuNqyXC4B2uVEPuaNvKnLTcAhGZqNQqJbb1FmNq20GJqrZI2Jm6rCSqx3ojqIIV5NJ-3QuMgd7RmLXbXgieuyHlG7NUjl3tH3UyUt-7Wduc5APxi39vIdiQAawhtoL5QMyRXxcHDDczvWfH1J9Rtf-qEu-tOM7rQYRmUxs8F6r9bON4qGxrGnptq6AFfPzJ41ywg13j9CWetsvB~hGcyMp2hdvThF97WMn-BSkFo4Kw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal