Lysyl oxidase (LOX) is a facilitator of extracellular matrix cross-linking. Using newly developed megakaryocyte-specific LOX knockout mice, we show that LOX expressed in these scarce bone marrow cells affects bone volume and collagen architecture in a sex-dependent manner.
TO THE EDITOR:
Dysregulated expression of lysyl oxidase (LOX) has been associated with several diseases.1 LOX is a copper-dependent secreted enzyme that stabilizes the extracellular matrix by facilitating the cross-linking of collagen and elastin in the extracellular matrix.2 We reported LOX to be expressed in low-ploidy megakaryocytes (MKs) and nearly absent in high-ploidy ones.3 Upregulated LOX expression was observed in MKs of mice and patients with primary myelofibrosis,3,4 a myeloproliferative neoplasm with increased number of MKs and bone alterations.5 The importance of LOX in bone formation has been addressed in both in vitro and in vivo studies.6-14 Although MKs have been shown to interact with and affect osteoblasts,15,16 the influence of MK LOX on bone has not been elucidated. Here, we sought to investigate this effect by using mice in which LOX is specifically deleted in MKs with the Cre-LoxP system.
We first confirmed the deletion of LOX in MKs (supplemental Figure 1, available on the Blood website), and these knockout (KO) mice are referred to as Pf4Cre+/+LOXfl/fl. Our previous study showed that an irreversible inhibitor of LOX, β-aminopropionitrile (BAPN), did not affect MK ploidy, but modestly reduced MK number in a mouse culture.3 Our current in vivo studies show no significant difference in complete blood cell count, number of hematopoietic stem cells, MK number and ploidy profile, and MK distribution in the bone marrow between Pf4Cre+/+LOXfl/fl and age- and sex-matched control Pf4Cre+/+ mice (supplemental Figures 2-4; supplemental Table 1).
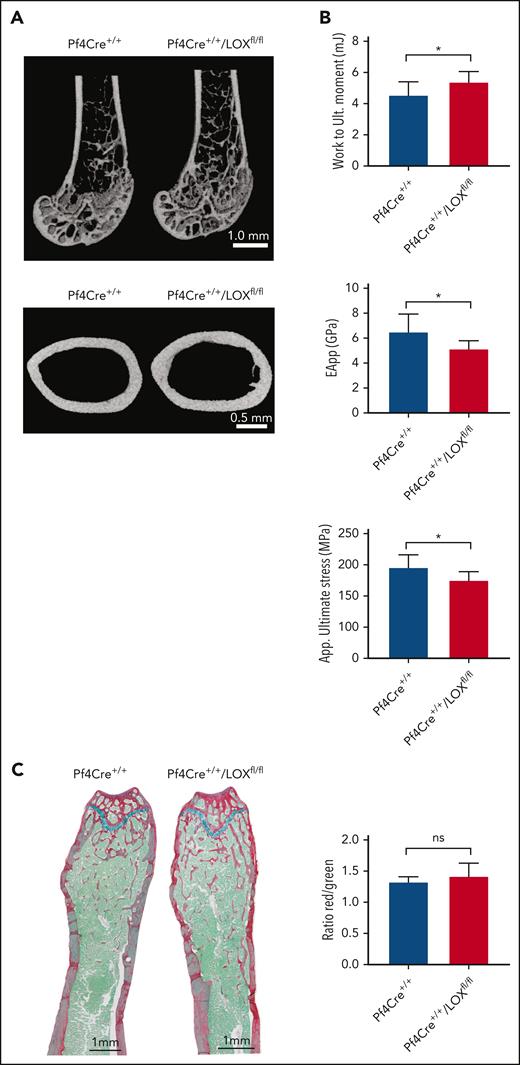
To test a possible effect of MK LOX on bone, we performed micro–computed tomography of mouse femurs. Mature male Pf4Cre+/+LOXfl/fl mice (17 and 32-37 weeks old) had significant changes in most bone parameters tested, such as both trabecular and cortical bone properties, showing greater trabecular bone volume fraction and trabecular number, as well as greater cortical and total area, and moments of inertias (polar moment of inertia [pMOI], maximum [Imax],and minimum [Imin]) at the middiaphysis, compared with matching controls (Figure 1A; supplemental Table 2). No significant changes were observed between the female experimental groups (supplemental Figure 5; supplemental Table 2), suggesting possible sex hormone–related differences. Although the femurs from male Pf4Cre+/+LOXfl/fl mice had greater cortical area and moments of inertias, which would all be predictors of having greater mechanical properties than their control counterparts, their apparent material properties were diminished compared with control femurs, which is shown by their significantly lower apparent bending modulus and ultimate stress (Figure 1B; supplemental Figure 6). The greater bone area in these femurs still allowed the bone of Pf4Cre+/+/LOXfl/fl mice to absorb significantly more work up to the ultimate moment point (Figure 1B, upper panel). There was no difference between male Pf4Cre+/+LOXfl/fl mice and their controls regarding the amount of osteoid,17 which is the unmineralized organic portion of the bone matrix that forms before the maturation of bone tissue (Figure 1C). This suggested that MK LOX does not have a significant effect on osteoblast differentiation.
LOX deletion in MKs leads to changes in bone volume and mechanical strength in male mice. (A) Representative micro–computed tomography (μCT) images from femurs of Pf4Cre+/+/LOXfl/fl mice and matching controls. The upper panel shows a distal femoral metaphysis, and the lower panel shows a femoral middiaphysis. Nine Pf4Cre+/+/LOXfl/fl and 7 Pf4Cre+/+ 17-week-old male mice were analyzed. (B) Three-point bending of the femoral diaphysis from the same femurs used for μCT and histology. This assay determines bone mechanical properties. Work to maximum moment (mJ), apparent bending modulus (GPa), and apparent ultimate stress (MPa) are shown. The rest of the 3-point bending parameters can be found in supplemental Figure 6. Plots are mean ± SD for the above number of mice. ∗P < .05. (C) Quantitative analysis of the ratio osteoid (red)/mineralized bone (green) for trabecular bones. Femurs of Pf4Cre+/+/LOXfl/fl mice and matching controls were stained with picrosirius red, fast green, and alcian blue, providing discrimination between mineralized and unmineralized (osteoid) bone. Osteoid appears red, mineralized bone appears green, and cartilage appears blue. Measurements are presented as mean ± SD. Analysis of the trabecular bone was conducted 50 μm distant from the edge of the growth plate, extending 300 μm. Four Pf4Cre+/+/LOXfl/fl and 4 Pf4Cre+/+ 17-week-old male mice were analyzed. EApp, apparent modulus of elasticity; ns, not significant; Work to Ult moment, work to ultimate momentum.
LOX deletion in MKs leads to changes in bone volume and mechanical strength in male mice. (A) Representative micro–computed tomography (μCT) images from femurs of Pf4Cre+/+/LOXfl/fl mice and matching controls. The upper panel shows a distal femoral metaphysis, and the lower panel shows a femoral middiaphysis. Nine Pf4Cre+/+/LOXfl/fl and 7 Pf4Cre+/+ 17-week-old male mice were analyzed. (B) Three-point bending of the femoral diaphysis from the same femurs used for μCT and histology. This assay determines bone mechanical properties. Work to maximum moment (mJ), apparent bending modulus (GPa), and apparent ultimate stress (MPa) are shown. The rest of the 3-point bending parameters can be found in supplemental Figure 6. Plots are mean ± SD for the above number of mice. ∗P < .05. (C) Quantitative analysis of the ratio osteoid (red)/mineralized bone (green) for trabecular bones. Femurs of Pf4Cre+/+/LOXfl/fl mice and matching controls were stained with picrosirius red, fast green, and alcian blue, providing discrimination between mineralized and unmineralized (osteoid) bone. Osteoid appears red, mineralized bone appears green, and cartilage appears blue. Measurements are presented as mean ± SD. Analysis of the trabecular bone was conducted 50 μm distant from the edge of the growth plate, extending 300 μm. Four Pf4Cre+/+/LOXfl/fl and 4 Pf4Cre+/+ 17-week-old male mice were analyzed. EApp, apparent modulus of elasticity; ns, not significant; Work to Ult moment, work to ultimate momentum.
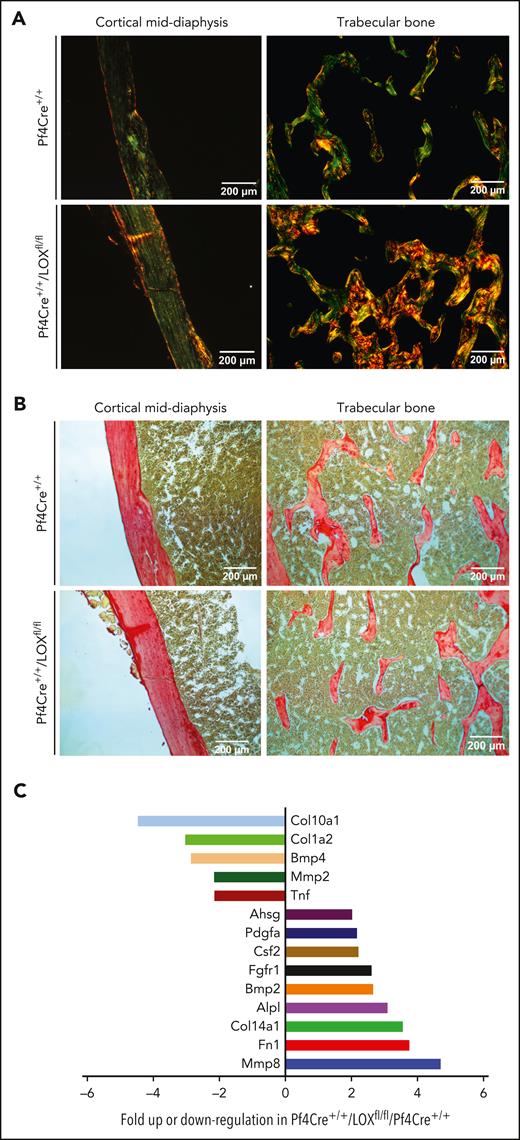
Because mechanical assessment of the bone pointed to compromised material properties in MK LOX KO mice compared with controls, and because LOX, a promoter of matrix cross-linking, has been studied in the context of fibrotic disorders, and its inhibition reduced collagen cross-linking,1,18 we assessed differences in collagen deposition. This was performed using picrosirius red staining, and visualization under polarized and nonpolarized light. Picric acid can more easily dissolve into collagen fibers that are loosely packed. When picric acid penetrates collagen, a bright yellow birefringence is observed under polarized light, as clearly seen in the male Pf4Cre+/+/LOXfl/fl mice (Figure 2A). When the stain is less able to penetrate the collagen because of tight fibril packing, the collagen profile, as seen in the control mice, appears less bright under polarized light, with birefringence mixed with evidence of green, yellow, and sometimes orange/red (Figure 2A). Quantification of total level of collagen within bone sections seemed similar in the Pf4Cre+/+/LOXfl/fl mice, compared with controls (Figure 2B; supplemental Figure 7), as also confirmed by a biochemical assay of hydroxyproline (supplemental Figure 8). These results indicate that collagen organization, and likely not overall collagen content, is disrupted in the Pf4Cre+/+/LOXfl/fl male mice compared with controls.
LOX deletion in male MKs impacts bone collagen structure but not level, with associated changes in MK gene expression. (A) Representative images of bone sections of Pf4Cre+/+ and Pf4Cre+/+/LOXfl/fl male mice stained with picrosirius red and visualized under polarized light. Picric acid can more easily dissolve into collagen fibers that are loosely packed, yielding a bright yellow birefringence, as observed under polarized light. When the stain is less able to penetrate collagen because of tight fibril packing, collagen profile appears less bright under polarized light, with birefringence mixed with evidence of green, yellow, and sometimes orange/red. Five Pf4Cre+/+/LOXfl/fl and 3 Pf4Cre+/+ 17-week-old male mice were analyzed. Images shown are representative of at least 3 slides analyzed in each case. (B) Representative images of bone sections of Pf4Cre+/+ and Pf4Cre+/+/LOXfl/fl male mice stained with picrosirius red and visualized under nonpolarized light for total collagen detection. Five Pf4Cre+/+/LOXfl/fl and 3 Pf4Cre+/+ 17-week-old male mice were analyzed. Images shown are representative of at least 3 slides analyzed in each case. (C) Differential expression of bone development–related genes in MKs of Pf4Cre+/+/LOXfl/fl mice compared with Pf4Cre+/+ mice with a graphical representation of >twofold difference in expression, using the commercial RT2 Profiler PCR Array Mouse Osteogenesis PAMM-026Z. mRNA results were normalized to β-actin, β-2 microglobulin, glyceraldehyde-3-phosphate dehydrogenase, β-glucuronidase, and heat shock protein 90-α. Eight 12-week-old control mice and 8 12-week-old Pf4Cre+/+/LOXfl/fl mice were analyzed. MKs derived from 4 mice in each experimental group were combined as 1 sample to obtain sufficient material and increase biological representation. Ahsg, alpha-2-HS-glycoprotein; Alp, alkaline phosphatase; Bmp, bone morphogenetic protein; COl, collagen; Csf, colony stimulating factor; Fgfr, fibroblast growth factor receptor; Fn, fibronectin; Mmp, matrix metallopeptidase; Pdgfa, platelet derived growth factor alpha; Tnf, tumor necrosis factor.
LOX deletion in male MKs impacts bone collagen structure but not level, with associated changes in MK gene expression. (A) Representative images of bone sections of Pf4Cre+/+ and Pf4Cre+/+/LOXfl/fl male mice stained with picrosirius red and visualized under polarized light. Picric acid can more easily dissolve into collagen fibers that are loosely packed, yielding a bright yellow birefringence, as observed under polarized light. When the stain is less able to penetrate collagen because of tight fibril packing, collagen profile appears less bright under polarized light, with birefringence mixed with evidence of green, yellow, and sometimes orange/red. Five Pf4Cre+/+/LOXfl/fl and 3 Pf4Cre+/+ 17-week-old male mice were analyzed. Images shown are representative of at least 3 slides analyzed in each case. (B) Representative images of bone sections of Pf4Cre+/+ and Pf4Cre+/+/LOXfl/fl male mice stained with picrosirius red and visualized under nonpolarized light for total collagen detection. Five Pf4Cre+/+/LOXfl/fl and 3 Pf4Cre+/+ 17-week-old male mice were analyzed. Images shown are representative of at least 3 slides analyzed in each case. (C) Differential expression of bone development–related genes in MKs of Pf4Cre+/+/LOXfl/fl mice compared with Pf4Cre+/+ mice with a graphical representation of >twofold difference in expression, using the commercial RT2 Profiler PCR Array Mouse Osteogenesis PAMM-026Z. mRNA results were normalized to β-actin, β-2 microglobulin, glyceraldehyde-3-phosphate dehydrogenase, β-glucuronidase, and heat shock protein 90-α. Eight 12-week-old control mice and 8 12-week-old Pf4Cre+/+/LOXfl/fl mice were analyzed. MKs derived from 4 mice in each experimental group were combined as 1 sample to obtain sufficient material and increase biological representation. Ahsg, alpha-2-HS-glycoprotein; Alp, alkaline phosphatase; Bmp, bone morphogenetic protein; COl, collagen; Csf, colony stimulating factor; Fgfr, fibroblast growth factor receptor; Fn, fibronectin; Mmp, matrix metallopeptidase; Pdgfa, platelet derived growth factor alpha; Tnf, tumor necrosis factor.
In searching for genes that could affect the extracellular matrix and bone formation, an mRNA mouse osteogenesis array including 84 transcripts was performed using flow cytometry sorted low-ploidy MKs (supplemental Table 3). We focused on these cells considering that LOX is scarce in control high-ploidy MKs. Differentially expressed transcripts in male Pf4Cre+/+/LOXfl/fl MKs vs Pf4Cre+/+ MKs with >twofold difference are shown in Figure 2C. Interestingly, of the displayed transcripts, matrix metallopeptidase 8 stood out with its nearly fivefold upregulated expression in Pf4Cre+/+/LOXfl/fl MKs compared with Pf4Cre+/+ MKs, as it is known for its involvement in the breakdown of the extracellular matrix.19 Also of interest is the downregulation of expression of collagen10a1 (Col10a1) in absence of LOX, because of the several known types of collagen, Col10a1 forms fibrils in the cartilage.20 However, this was not manifested in comparing total collagen levels in the experimental groups. Future studies could explore LOX regulation of these and other displayed genes. There was no difference in the expression of other LOX isoforms, such as LOXl1 and LOXl2, in Pf4Cre+/+/LOXfl/fl MKs vs Pf4Cre+/+ MKs from male animals (supplemental Figure 9).
Intriguing was the lack of bone phenotype in LOX KO female mice. We considered that this might relate to differences in LOX or LOX isoform expression in male and female MKs, which could present a greater reservoir of these enzymes in females. Indeed, LOXL1 and LOXL2 levels were reported to be higher in females than males.21 A transcriptome analysis in female mice was also performed (supplemental Figure 10). Contrary to results in male MKs, deletion of LOX led to downregulation of matrix metallopeptidase 9 and collagen4a1 in MKs, the effect of which might be balanced by upregulation of expression of genes, such as collagen1a1.
In other studies, 47-day-old female rats fed with a BAPN-containing diet (for 2 or 4 weeks) or 5-week-old male mice subcutaneously injected with BAPN (for 3 weeks) presented with reduced collagen cross-linking and trabecular bone and cortical bone fracture toughness and strength; however, the mineral properties, which are known to correlate with stiffness, were not affected.8,9 Ida et al10 performed bone analyses 4 weeks after an 8-week BAPN-containing diet in female C57BL/6J mice. Similar to our studies, bone volumes tended to increase and the number of osteoblasts per bone surface and osteoblast surface per bone surface significantly increased in the BAPN-treated groups.10 In the current report, we show, for the first time, an important role of MK LOX in determining bone volume and collagen architecture in male mice. This is of particular interest considering the relative rarity of bone marrow MKs, and yet, their known interaction with bone cells.15 These findings have greater implications, because increased number of low-ploidy MKs and various bone alterations have been reported in diseases, such as myeloproliferative neoplasm.5 Finally, LOX inhibition has proven to reduce disease burden in hematopoietic malignancies.18,22 However, our results with mouse models raise the possibility that long-term ablation of LOX for therapeutic purposes might impact bone quality in a sex-dependent manner.
All protocols were approved by the Boston University Medical Campus Institutional Animal Care and Use Committee.
Acknowledgments
This work was supported by National Institutes of Health (NIH), National Heart, Lung, and Blood Institute grants HL136363 and HL 158670 (K.R.), and by a pilot program grant from the Boston University Clinical and Translational Science Institute to K.R. and P.T. S.M. is supported by NIH, Office of Research Infrastructure Programs grants KO1 OD025290 and R03 OD031959.
Authorship
Contribution: K.R. and A.K. generated the hypotheses; A.K. designed and performed most of the experiments, analyzed data, and cowrote the manuscript, all with K.R.; A.I.K. examined the distribution of megakaryocytes in the bone area and performed flow cytometry experiments and analysis for the percentage of hematopoietic stem cell population in the bone marrow; D.B. performed micro–computed tomography and mechanical analysis; S.M. advised on experimental protocols, and performed flow cytometry experiments and analysis to investigate the number, maturity, and ploidy of megakaryocytes in the bone marrow; V.D. performed mouse genotyping and validation at the initial stage of generating the mouse line; P.C.T. and K.R. designed the lysyl oxidase floxed mouse line; and P.C.T. provided advice throughout the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katya Ravid, Boston University Chobanian and Avedisian School of Medicine, 700 Albany St, W-6, Boston, MA 02118; email: kravid@bu.edu.
References
Author notes
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal