Thrombotic thrombocytopenic purpura (TTP) is an uncommon but potentially fatal disorder with limited treatment options, particularly in the acute disease setting. In this issue of Blood, Muller and colleagues1 explore the therapeutic potential of targeting interleukin-1 (IL-1) levels.
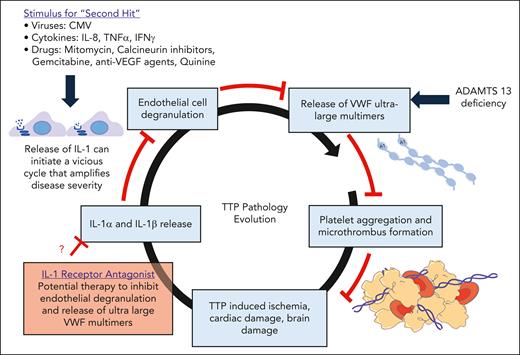
Patients with TTP experience severe thrombocytopenia, hemolytic anemia, and occlusion of blood vessels, leading to organ ischemia, predominantly in the heart and brain. TTP is associated with an autoantibody-mediated reduction in the activity and function of the von Willebrand factor (VWF) cleaving enzyme, a disintegrin-like and metalloproteinase with thrombospondin type 1 repeats 13 (ADAMTS13).2 As a result, ultralarge VWF multimers persist in the circulation, triggering microthrombus formation (see figure), leading to end organ damage.2
An IL-1–induced amplification loop in TTP. Release of ultralarge multimers in the absence of ADAMTS13 activity results in formation of platelet aggregates and microthrombi, causing ischemic injuries. This TTP-induced ischemia triggers the release of proinflammatory cytokines, such as IL-1, that can initiate damage, leading to amplification of disease severity. Anakinra-based IL-1 blockade is an appealing treatment that has been used effectively for a variety of autoimmune and inflammatory disease states and is effective in mitigating many pathophysiological features of TTP.
An IL-1–induced amplification loop in TTP. Release of ultralarge multimers in the absence of ADAMTS13 activity results in formation of platelet aggregates and microthrombi, causing ischemic injuries. This TTP-induced ischemia triggers the release of proinflammatory cytokines, such as IL-1, that can initiate damage, leading to amplification of disease severity. Anakinra-based IL-1 blockade is an appealing treatment that has been used effectively for a variety of autoimmune and inflammatory disease states and is effective in mitigating many pathophysiological features of TTP.
TTP mortality (fatal in >90% of cases) was drastically reduced by the introduction of plasma exchange; however, neurologic symptoms, including cognitive impairment and depression, are common in patients recovering from TTP and during relapse episodes.3 Although plasmapheresis with plasma exchange to deplete the offending antibody and replenish ADAMTS13 activity are preferred therapeutic options together with immunosuppression, typically with corticosteroids and anti-CD20 therapy, years of dedicated discovery research has provided several new and developing therapeutic alternatives. First, caplacizumab, a nanobody directed against the VWF A1 domain, which interferes with VWF-platelet interactions, is now incorporated into international guidelines for management of TTP.4 Second, and still under development, infusion of recombinant ADAMTS13 (rADAMTS13), including refined and improved preparations, where residues within ADAMTS13 critical for enzymatic activity and targeted by autoantibodies are modified, is an emerging therapy for TTP.5 Third, restoration of therapeutic levels of ADAMTS13 have been demonstrated in vitro, using platelets loaded with rADAMTS13 or in murine studies with lipid particles bearing messenger RNA encoding ADAMTS13 that were targeted to the liver. Other reports have described the beneficial effects of N-acetylcysteine therapy to modify VWF size and activity and resolve microthrombi, although N-acetylcysteine was later shown to be ineffective in treating preexisting signs of TTP in a baboon model.6 Pure discovery research, in this case focusing on understanding the pathophysiology of TTP, continues to provide exponential returns on research funding investments. However, in the current era of rituximab and caplacizumab, patients with TTP still develop adverse health consequences and have increased mortality on long-term follow-up.7 Cardiovascular disease remains one of the leading causes of death among TTP survivors.
The article at hand by Muller et al presents a wealth of discovery research data that suggest a pathologic role for IL-1 in relapsed disease and an improvement to ischemia indexes in ADAMTS13-deficient mice treated with an IL-1 receptor antagonist (see figure). The interleukin family and particularly isoforms IL-1α and IL-1β have been extensively investigated in the setting of inflammation, innate immune processes, and tissue ischemia.8 IL-1 family cytokines activate endothelial cells to release VWF and increase permeability.9 Here, the authors have identified a role for IL-1 in postischemic organ damage in TTP that was independent of the immune mechanism of the disease. Elevated levels of IL-1α and IL-1β were found in samples from patients with acute TTP compared with remission phase and control groups. These levels correlated with disease severity and mortality. The IL-1 findings were confirmed in a murine model of TTP, whereupon ADAMTS13-deficient mice were treated on 3 consecutive days with 1500 IU/kg recombinant human VWF, resulting in rapid onset of TTP symptoms, including severe thrombocytopenia, hemolytic anemia, and capillary microthrombi. Plasma IL-1α and IL-1β concentrations were modestly elevated, and mice exhibited cardiac and cerebral pathologies. Pretreatment with IL-1 receptor antagonist (RA), anakinra, previously shown to be effective in ischemia models, significantly reduced ischemic myocardial lesions and numbers of hypoxic Purkinje cells. In survival studies, ADAMTS13-deficient mice injected with 100 mg/kg anakinra showed improved survival, along with fewer microthrombi in capillaries of myocardial tissues compared with untreated ADAMTS13-deficient mice. Reduction in damage to cerebral tissues was also observed in these mice. To assess the effect of plasma IL-1, the authors exposed microvascular endothelial cells maintained in vitro, to exogenously added IL-1α, IL-1β, or TTP patient plasma, and demonstrated a calcium-dependent increase in VWF release that was blocked by the IL-1 RA in plasma-treated cultures. Finally, direct administration of IL-1α and IL-1β to ADAMTS13-deficient mice rapidly induced increased plasma VWF, microangiopathy, severe thrombocytopenia, and cardiac damage, thus providing a mechanistic link between IL-1 levels and TTP.
Anakinra is a recombinant form of the human IL-1 RA protein and is a leading anti–IL-1 therapeutic that blocks the proinflammatory effects of IL-1β, featuring a short half-life and acceptable safety profile. Anakinra is effective for treatment of autoimmune, inflammatory, infectious, metabolic, and malignant diseases.10 However, the contribution of IL-1 to TTP pathology has not been explored in depth, and so the association of IL-1 with endothelial consequences (enhanced VWF release) is an exciting new vascular link in TTP. The authors propose that in TTP, a prolonged ADAMTS13 deficiency triggers VWF release from cardiac cells and sensitizes them to IL-1 toxicity. IL-1 receptor blockade effectively reduced ischemic end organ damage, a feared outcome of TTP. A role for IL-1, or effectiveness of anakinra in combating earlier stages of TTP, was not explored because of the acute onset of TTP in the model selected. Further, the source of IL-1 in ADAMTS-13 mice with TTP symptoms and whether IL-1 levels reflect ongoing endothelial damage or play a critical role in TTP pathophysiology are open questions. Supraphysiological levels of IL-1 were required to elicit ischemic changes, hinting that IL-1 levels do not trigger TTP pathology; however, whether local levels of IL-1 induce subtle but important changes to microvascular behaviors remains to be addressed. Notably, treatment of ADAMTS13-deficient mice with VWF and anakinra did not alleviate hemoglobin or platelet symptoms in the TTP mice, indicating that more research into what triggers endothelial degranulation is required.
In conclusion, this work indicates that IL-1 contributes to ischemic injury and mortality in mouse models of TTP. Further work evaluating IL-1 blockade in conjunction with standard therapies, and improved understanding of the consequences of prolonged blockade of IL-1 receptor pathways, will progress and translate this exciting discovery research outcome to the clinic.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal