Visual Abstract
The direct oral anticoagulants (DOACs) rivaroxaban and dabigatran are newly licensed for the treatment and prevention of venous thromboembolism (VTE) in children and mark a renaissance in pediatric anticoagulation management. They provide a convenient option over standard-of-care anticoagulants (heparins, fondaparinux, and vitamin K antagonists) because of their oral route of administration, child-friendly formulations, and significant reduction in monitoring. However, limitations related to therapeutic monitoring when needed and the lack of approved reversal agents for DOACs in children raise some safety concerns. There is accumulating experience of safety and efficacy of DOACs in adults for a broad scope of indications; however, the cumulative experience of using DOACs in pediatrics, specifically for those with coexisting chronic illnesses, is sparse. Consequently, clinicians must often rely on their experience for treating VTE and extrapolate from data in adults while using DOACs in children. In this article, the authors share their experience of managing 4 scenarios that hematologists are likely to encounter in their day-to-day practice. Topics addressed include (1) appropriateness of indication; (2) use for special populations of children; (3) considerations for laboratory monitoring; (4) transition between anticoagulants; (5) major drug interactions; (6) perioperative management; and (7) anticoagulation reversal.
Introduction
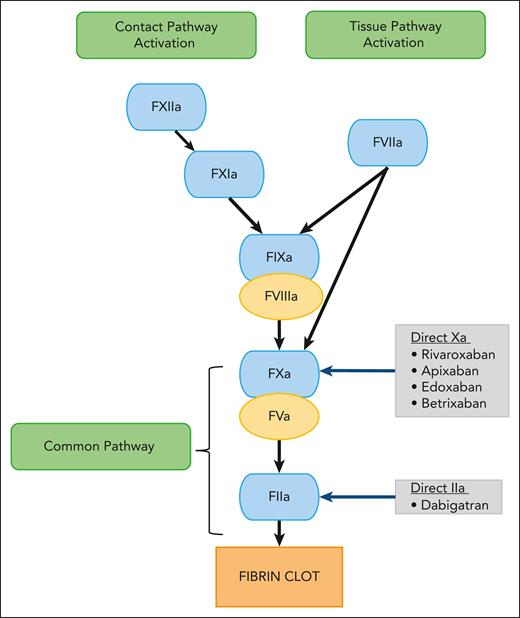
Direct oral anticoagulants (DOACs) have led to a paradigm shift in venous thromboembolism (VTE) management. These small molecules reversibly and directly inhibit specific coagulation enzymes (Figure 1).1 Currently, 2 classes of DOACs are available: the direct factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban) and the direct factor IIa (thrombin) inhibitor (dabigatran).2-4 Their oral route of administration is an attractive option over standard-of-care (SOC) parenteral anticoagulants, such as low molecular weight heparin (LMWH), unfractionated heparin (UFH), and fondaparinux. Furthermore, other advantages over oral SOC vitamin K antagonists include stable pharmacokinetics (PK) and pharmacodynamics, wide therapeutic window, availability of child-friendly formulations, immediate onset and offset of action, minimal or no monitoring, and fewer drug and food interactions3,5,6 Thus, DOACs have significant advantages compared with SOC agents.7-9
Schematic diagram showing the mechanism of action of DOACs. F, factor.
Since the approval of DOACs in adults in 2008,10 the off-label use of DOACs in children for treatment of VTE has been increasing even before their pediatric approval in 2021.2,8,10-13 With the completed HOKUSAI-Jr phase 3 (edoxaban)14 and ongoing CANINES phase 4 (apixaban) trials,15 the choice of anticoagulants will broaden. In addition to VTE treatment, DOACs have been evaluated for VTE prevention in children with congenital and acquired heart disease and cancer, thereby adding to the growing number of children enrolled in clinical trials (Table 1).16-18,22-25
Completed DOAC phase 2b/3 pediatric thrombosis trials
| Trial/phase . | Indication . | Age group . | Comparator/SOC agent . | Initial treatment . | No. of children treated . | Outcomes . | Key observations . |
|---|---|---|---|---|---|---|---|
| Rivaroxaban∗ | |||||||
| Einstein Jr phase 316 (NCT02234843) | VTE treatment and prevention of recurrent VTE | From birth to age <18 y | SOC (UFH, LMWH, fondaparinux, and VKA) | ≥5 d of SOC anticoagulant | 500 | Rivaroxaban vs SOC anticoagulant Efficacy: symptomatic recurrent VTE: 4 (1%) vs 5 (3%); HR, 0.4; 95% CI, 0.11-1.41. Safety: major bleeding/CRNMB: 10 (3%) (all nonmajor) vs 3 (2%) (1 nonmajor and 1 CRNMB); HR, 1.58; 95% CI, 0.51-6.27. | Patients received SOC anticoagulant for 5-9 d before starting rivaroxaban. CVC-provoked VTE represented 25% of study population. Infants and younger children were underrepresented (37 of 335 [11%]). Subanalysis of special populations reported: CVC, infection-related CSVT, and cancer. |
| UNIVERSE phase 317 (NCT02846532) | Thromboprophylaxis for children after Fontan procedure | 2-8 y | Part A: none Part B: aspirin | NA | 112 | Part B: rivaroxaban vs aspirin Efficacy: event rate, 2 (3%) vs 3 (9%) Safety: major bleeding, 1 (2%) in rivaroxaban CRNMB: 4 (6%) vs 3 (9%) | Shorter duration between Fontan surgery and the first study drug dose in the aspirin group (mean, 37 d) than in the rivaroxaban group (mean, 45 d). Not powered to test a formal hypothesis for efficacy. |
| Apixaban | |||||||
| PREVAPIX-ALL phase 318,19 (NCT02369653) | Thromboprophylaxis during induction chemotherapy for ALL/LL | 1-18 y | None | NA | 512 | Apixaban vs SOC anticoagulant† Efficacy: VTE occurrence, 31 (12.1%) vs 45 (17.6%); RR, 0.69 (0.45-1.05); 1-sided P = .04 Safety: major bleeding, 2 in each arm; CRNMB, 11 vs 3 events | Apixaban was not shown to be efficacious in the primary analysis but decreased VTE risk for patients with obesity The study design was powered to demonstrate the benefit of anticoagulant prophylaxis of CVL-associated thrombosis for children with ALL/LL. |
| SAXOPHONE phase 220 (NCT03395639) | Thromboprophylaxis for cardiac disease | From 29 d to <18 y of age | SOC anticoagulant (LMWH or VKA) | NA | 192 | Apixaban vs SOC anticoagulant† Efficacy: no thromboembolic (TE) events in either arm. Safety: 1 had 2 primary safety events (IR, 1.8/100 P-Y) vs 3 with 4 events (IR, 6.8/100 P-Y). | Bone density and quality of life were measured for 12 mo but not reported. |
| Edoxaban | |||||||
| ENOBLE phase 321 NCT02798471 | Thromboprophylaxis in cardiac disease | 38 wk to <18 y | SOC (UFH, LMWH, VKA) | NA | 168 | Edoxaban vs SOC anticoagulant Efficacy: none vs 2 TE events in SOC (1.7%) Safety: major, none; CRNMB, 1 in each group. Extension arm (n = 147, all on edoxaban) Efficacy: 4 TE (2.8%; 2 strokes and 2 coronary artery thrombosis or myocardial infarction) Safety: major, none; CRNMB, 1 (0.7%). | Compliance with investigational drug was measured and was 94% in the edoxaban group in the main treatment period but reduced to 55% in the extension study. |
| HOKUSAI-Jr phase 314 (NCT02798471) | VTE treatment | From 38 wk to <18 y of age | SOC (UFH, LMWH, fondaparinux, and VKA) | ≥5 d of parenteral treatment | 290 | Not available | Study completed; study results not published. |
| Dabigatran∗ | |||||||
| DIVERSITY phase 2b/322 (NCT01895777) | VTE treatment and prevention of recurrent VTE | From birth to age 17 y | SOC anticoagulant (LMWH, VKA, or fondaparinux) | ≥5 d of parenteral treatment | 260 | Dabigatran vs SOC anticoagulant Efficacy: Composite outcome: 81 (46%) vs 38 (42%); P = .001 Safety: on treatment bleeding, 38/176 (22%) vs 22/90 (24%); HR, 1·15; 95% CI, 0·68-1·94; P = ·61 Major bleeding: 4/176 (2%) vs 2/90 (2%); HR, 0·94; 95% CI, 0·17-5·16; P = ·95 | Patients received 5-21 d of SOC anticoagulant before starting dabigatran. Dabigatran drug levels were monitored to determine appropriate dose. 17 of 176 (∼10%) of the population prematurely discontinued dabigatran because of failure to achieve target dabigatran plasma concentration after 1 dose adjustment allowed per protocol. Infants and younger children were underrepresented (22/176 [12.5%]). Subanalysis of special populations: CVC, CSVT, and thrombophilia from birth to <2 y of age. |
| DIVERSITY phase 38 NCT 02197416 | VTE secondary prevention (single arm) | From birth to <18 y of age | NA | NA | 203 | Efficacy: 1% (2/203) recurrence Safety: major bleeding, 1.5% (3/203). CRNMB, 1% (2/203). | Study reported development of postthrombotic syndrome in 2 of 162 participants (1.2%) with DVT- or CVC-related thrombosis. |
| Trial/phase . | Indication . | Age group . | Comparator/SOC agent . | Initial treatment . | No. of children treated . | Outcomes . | Key observations . |
|---|---|---|---|---|---|---|---|
| Rivaroxaban∗ | |||||||
| Einstein Jr phase 316 (NCT02234843) | VTE treatment and prevention of recurrent VTE | From birth to age <18 y | SOC (UFH, LMWH, fondaparinux, and VKA) | ≥5 d of SOC anticoagulant | 500 | Rivaroxaban vs SOC anticoagulant Efficacy: symptomatic recurrent VTE: 4 (1%) vs 5 (3%); HR, 0.4; 95% CI, 0.11-1.41. Safety: major bleeding/CRNMB: 10 (3%) (all nonmajor) vs 3 (2%) (1 nonmajor and 1 CRNMB); HR, 1.58; 95% CI, 0.51-6.27. | Patients received SOC anticoagulant for 5-9 d before starting rivaroxaban. CVC-provoked VTE represented 25% of study population. Infants and younger children were underrepresented (37 of 335 [11%]). Subanalysis of special populations reported: CVC, infection-related CSVT, and cancer. |
| UNIVERSE phase 317 (NCT02846532) | Thromboprophylaxis for children after Fontan procedure | 2-8 y | Part A: none Part B: aspirin | NA | 112 | Part B: rivaroxaban vs aspirin Efficacy: event rate, 2 (3%) vs 3 (9%) Safety: major bleeding, 1 (2%) in rivaroxaban CRNMB: 4 (6%) vs 3 (9%) | Shorter duration between Fontan surgery and the first study drug dose in the aspirin group (mean, 37 d) than in the rivaroxaban group (mean, 45 d). Not powered to test a formal hypothesis for efficacy. |
| Apixaban | |||||||
| PREVAPIX-ALL phase 318,19 (NCT02369653) | Thromboprophylaxis during induction chemotherapy for ALL/LL | 1-18 y | None | NA | 512 | Apixaban vs SOC anticoagulant† Efficacy: VTE occurrence, 31 (12.1%) vs 45 (17.6%); RR, 0.69 (0.45-1.05); 1-sided P = .04 Safety: major bleeding, 2 in each arm; CRNMB, 11 vs 3 events | Apixaban was not shown to be efficacious in the primary analysis but decreased VTE risk for patients with obesity The study design was powered to demonstrate the benefit of anticoagulant prophylaxis of CVL-associated thrombosis for children with ALL/LL. |
| SAXOPHONE phase 220 (NCT03395639) | Thromboprophylaxis for cardiac disease | From 29 d to <18 y of age | SOC anticoagulant (LMWH or VKA) | NA | 192 | Apixaban vs SOC anticoagulant† Efficacy: no thromboembolic (TE) events in either arm. Safety: 1 had 2 primary safety events (IR, 1.8/100 P-Y) vs 3 with 4 events (IR, 6.8/100 P-Y). | Bone density and quality of life were measured for 12 mo but not reported. |
| Edoxaban | |||||||
| ENOBLE phase 321 NCT02798471 | Thromboprophylaxis in cardiac disease | 38 wk to <18 y | SOC (UFH, LMWH, VKA) | NA | 168 | Edoxaban vs SOC anticoagulant Efficacy: none vs 2 TE events in SOC (1.7%) Safety: major, none; CRNMB, 1 in each group. Extension arm (n = 147, all on edoxaban) Efficacy: 4 TE (2.8%; 2 strokes and 2 coronary artery thrombosis or myocardial infarction) Safety: major, none; CRNMB, 1 (0.7%). | Compliance with investigational drug was measured and was 94% in the edoxaban group in the main treatment period but reduced to 55% in the extension study. |
| HOKUSAI-Jr phase 314 (NCT02798471) | VTE treatment | From 38 wk to <18 y of age | SOC (UFH, LMWH, fondaparinux, and VKA) | ≥5 d of parenteral treatment | 290 | Not available | Study completed; study results not published. |
| Dabigatran∗ | |||||||
| DIVERSITY phase 2b/322 (NCT01895777) | VTE treatment and prevention of recurrent VTE | From birth to age 17 y | SOC anticoagulant (LMWH, VKA, or fondaparinux) | ≥5 d of parenteral treatment | 260 | Dabigatran vs SOC anticoagulant Efficacy: Composite outcome: 81 (46%) vs 38 (42%); P = .001 Safety: on treatment bleeding, 38/176 (22%) vs 22/90 (24%); HR, 1·15; 95% CI, 0·68-1·94; P = ·61 Major bleeding: 4/176 (2%) vs 2/90 (2%); HR, 0·94; 95% CI, 0·17-5·16; P = ·95 | Patients received 5-21 d of SOC anticoagulant before starting dabigatran. Dabigatran drug levels were monitored to determine appropriate dose. 17 of 176 (∼10%) of the population prematurely discontinued dabigatran because of failure to achieve target dabigatran plasma concentration after 1 dose adjustment allowed per protocol. Infants and younger children were underrepresented (22/176 [12.5%]). Subanalysis of special populations: CVC, CSVT, and thrombophilia from birth to <2 y of age. |
| DIVERSITY phase 38 NCT 02197416 | VTE secondary prevention (single arm) | From birth to <18 y of age | NA | NA | 203 | Efficacy: 1% (2/203) recurrence Safety: major bleeding, 1.5% (3/203). CRNMB, 1% (2/203). | Study reported development of postthrombotic syndrome in 2 of 162 participants (1.2%) with DVT- or CVC-related thrombosis. |
CRNMB, clinically relevant nonmajor bleeding; CVC, central venous catheter; HR, hazard ratio; NA, not applicable; LL, lymphoblastic lymphoma; VKA, vitamin K antagonists.
Approved in North America and the United Kingdom and by the European Medicines Agency.
Abstracts at International Society of Thrombosis and Hemostasis Congress annual meeting, 2022.
Despite this positive development, some of the major limitations of the pediatric randomized clinical trials were underrepresentation or exclusion of special populations at high risk of thrombosis, such as neonates and young children, and those with cancer, renal and liver failure.2,16,24,26 Although DOACs do not routinely require therapeutic monitoring, the limited access to such testing and their reversal agents are a few disadvantages.27,28 Despite these limitations, the use of DOACs for children is rapidly increasing.
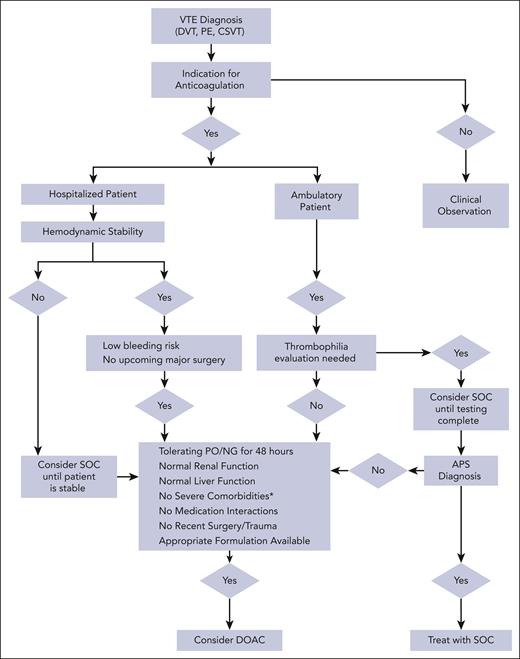
This article aims to provide practical recommendations to clinicians treating pediatric VTE, particularly for clinical scenarios in which DOACs can be used despite limited data. Before considering DOAC therapy, it is important to first critically assess the need for anticoagulation (Figure 2).29-31 Once the need for anticoagulation is confirmed, the authors approach each scenario with 3 questions before considering DOAC therapy: (1) is this patient a candidate for DOAC therapy? (2) what safety concerns require a priori attention before initiating a DOAC? and (3) what other practical considerations are pertinent to patient management. We present 4 VTE cases agewise, from a newborn to a teenager, highlighting age-specific challenges with anticoagulation.
Algorithm for pediatric VTE anticoagulation management. ∗indicates severe liver or renal dysfunction, short gut syndrome, severe thrombocytopenia, ICH, or postoperative or severe trauma. VTE, venous thromboembolism; SOC, standard of care; DOAC, direct oral anticoagulant; NG, nasogastric; PO, by mouth.
Algorithm for pediatric VTE anticoagulation management. ∗indicates severe liver or renal dysfunction, short gut syndrome, severe thrombocytopenia, ICH, or postoperative or severe trauma. VTE, venous thromboembolism; SOC, standard of care; DOAC, direct oral anticoagulant; NG, nasogastric; PO, by mouth.
Case 1: neonatal VTE
A 3-week-old infant born at 34 weeks of gestation weighing 3 kg developed a left iliofemoral deep venous thrombosis (DVT) secondary to a peripherally inserted central catheter (PICC). The infant had a grade 2 intracranial hemorrhage (ICH) diagnosed at birth and was on mechanical ventilation. The PICC cannot be removed because of the need for vascular access. Complete blood count, comprehensive metabolic panel, and coagulation testing were normal. The infant was tolerating transpyloric feeds.
Question 1: is this patient a candidate for DOAC therapy?
First, one must weigh the risk of bleeding vs the risk of DVT extension because of continued exposure to VTE risk factors (central venous catheter, mechanical ventilation, and preterm birth).32,33 Second, neonates and young children constituted a small proportion in the Einstein Jr and Diversity trials (∼10%).16,22 Third, there are no dosing nomograms for preterm (<37 weeks) infants.2 However, this infant is now of 37-week corrected gestational age, has normal liver and kidney functions, and is tolerating enteral feeding. Because the ICH is still within 30 days, an exclusion criterion for DOAC trials, it is safer to start treatment with an SOC agent, either UFH or LMWH, and monitor the ICH before considering a DOAC.
Question 2: what safety concerns require a priori attention before initiating a DOAC?
Because this patient is a neonate, the risk of bleeding or ICH progression deserves careful discussion. In the DOAC clinical trials, the risk of major bleeding and clinically relevant nonmajor bleeding was from 1% to 2% (Table 1). In the Diversity trial, fewer children from birth to age <2 years experienced serious adverse events in the dabigatran arm (9%) than those aged 12 to <18 years (14%). In the same age group, 6 of 22 children (27%) receiving dabigatran experienced bleeding, of which 1 was a major ICH in an infant aged 1 month with meningitis. Most of the bleeding events reported were minor gastrointestinal (GI) bleeding (5%), epistaxis (5%), and bruising (3%).22,34 In the Einstein Jr trial, none of the children in the rivaroxaban arm (n = 329) experienced major bleeding.16,34 Nevertheless, it is prudent to consider initial treatment with SOC agent and monitor ICH before considering a DOAC. The second step is to carefully review treatment of other comorbidities that could contribute to the risk of bleeding. For instance, this infant is being treated for sepsis; therefore, drug-drug interactions must be reviewed, specifically whether there is coadministration of inducers and inhibitors of CYP3A4 and/or P-glycoprotein (P-gp) (Table 2).4,35 Additionally, critically ill infants frequently undergo invasive procedures; therefore, clinicians must provide specific recommendations about periprocedure management (Figure 3). The DOAC can be restarted after the risk of bleeding is low and the patient is able to tolerate oral intake.
Pharmacologic properties of DOAC agents
| Variable . | Dabigatran etexilate . | Rivaroxaban . | Apixaban∗ . | Edoxaban∗ . |
|---|---|---|---|---|
| Prodrug | Yes | No | No | No |
| Mechanism of action | Direct IIa inhibitor; inhibits clot-bound and free thrombin | Direct Xa inhibitor; inhibits clot-bound and free Xa | Direct Xa inhibitor; inhibits clot-bound and free Xa | Direct Xa inhibitor; inhibits clot-bound and free Xa |
| Time to onset of action and peak concentration | 22 min-4.5 h | 1-3 h | 1-2 h | 1-2 h |
| Oral bioavailability | 3%-7% | 66% (fasting); 80%-100% (with food) | 50%; prolonged absorption | 62% |
| Half-life | 12-17 h | 5-9 h | 8-12 h | 10-14 h |
| Plasma protein binding | 92%-95% | 87% | 99% | 55% |
| Metabolism | Conjugation, prodrug is P-gp substrate | CYP3A4/5, CYP2J2, hydrolysis, and P-gp substrate | CYP3A4 (major), CYP1A2, 2C8, 2C19, 2J2 (all minor), and P-gp substrate | Conjugation, hydrolysis, CYP3A4 (all minor), and P-gp substrate |
| Elimination | Renal (80%) | Renal (66%), fecal (7%), and unchanged (36%) | Renal (27%), fecal (56%), and biliary (minimal) | Renal (50%), metabolism and biliary/intestinal excretion (50%) |
| Absorption | Lower gastric region and duodenum | Primarily proximal small intestine and some gastric absorption | Primarily proximal small intestine and some gastric absorption | Primarily proximal small intestine and some gastric absorption |
| Antidote | Idarucizumab∗ | Andexanet-α∗ | Andexanet-α∗ | Andexanet-α∗ |
| Other options for overdose | Hemodialysis and gastric lavage with charcoal (within 2 h of consumption) | PCC (3 or 4 factor) | PCC (3 or 4 factor) | PCC (3 or 4 factor) and TXA |
| Food interaction | None | None | None | None |
| Drug interactions that increase drug levels | Amiodarone, quinidine, azole antifungals (eg, ketoconazole), and ritonavir proton pump inhibitor | Azole antifungals (eg, ketoconazole), all HIV protease inhibitors (eg, ritonavir), and clarithromycin | Azole antifungals (eg, ketoconazole), all HIV protease inhibitors (eg, ritonavir), and clarithromycin | Azole antifungals (eg, ketoconazole), all HIV protease inhibitors (eg, ritonavir), and clarithromycin |
| Drug interactions that decrease drug levels | Rifampin, phenytoin, carbamazepine, and St. John’s wort | Anticonvulsants (eg, phenytoin and carbamazepine), and rifampin | Anticonvulsants (phenytoin and carbamazepine), and rifampin | Anticonvulsants (phenytoin and carbamazepine), and rifampin |
| Laboratory measurement to assess anticoagulant effect† | aPTT, TCT, and dilute TCT | PT/INR and anti–factor Xa assay (for Xa inhibitor) | PT/INR [minimal effect] and anti–factor Xa assay (for Xa inhibitor) | Anti–factor Xa assay (for Xa inhibitor) |
| Available formulations | Capsules, pellets (sprinkles), and oral solution‡ | Tablet and oral solution | Tablet and oral solution | Tablet and oral solution |
| Patient assistance program for drug | Boehringer-Ingelheim | Johnson & Johnson | Bristol Myers Squibb | No program currently available |
| Variable . | Dabigatran etexilate . | Rivaroxaban . | Apixaban∗ . | Edoxaban∗ . |
|---|---|---|---|---|
| Prodrug | Yes | No | No | No |
| Mechanism of action | Direct IIa inhibitor; inhibits clot-bound and free thrombin | Direct Xa inhibitor; inhibits clot-bound and free Xa | Direct Xa inhibitor; inhibits clot-bound and free Xa | Direct Xa inhibitor; inhibits clot-bound and free Xa |
| Time to onset of action and peak concentration | 22 min-4.5 h | 1-3 h | 1-2 h | 1-2 h |
| Oral bioavailability | 3%-7% | 66% (fasting); 80%-100% (with food) | 50%; prolonged absorption | 62% |
| Half-life | 12-17 h | 5-9 h | 8-12 h | 10-14 h |
| Plasma protein binding | 92%-95% | 87% | 99% | 55% |
| Metabolism | Conjugation, prodrug is P-gp substrate | CYP3A4/5, CYP2J2, hydrolysis, and P-gp substrate | CYP3A4 (major), CYP1A2, 2C8, 2C19, 2J2 (all minor), and P-gp substrate | Conjugation, hydrolysis, CYP3A4 (all minor), and P-gp substrate |
| Elimination | Renal (80%) | Renal (66%), fecal (7%), and unchanged (36%) | Renal (27%), fecal (56%), and biliary (minimal) | Renal (50%), metabolism and biliary/intestinal excretion (50%) |
| Absorption | Lower gastric region and duodenum | Primarily proximal small intestine and some gastric absorption | Primarily proximal small intestine and some gastric absorption | Primarily proximal small intestine and some gastric absorption |
| Antidote | Idarucizumab∗ | Andexanet-α∗ | Andexanet-α∗ | Andexanet-α∗ |
| Other options for overdose | Hemodialysis and gastric lavage with charcoal (within 2 h of consumption) | PCC (3 or 4 factor) | PCC (3 or 4 factor) | PCC (3 or 4 factor) and TXA |
| Food interaction | None | None | None | None |
| Drug interactions that increase drug levels | Amiodarone, quinidine, azole antifungals (eg, ketoconazole), and ritonavir proton pump inhibitor | Azole antifungals (eg, ketoconazole), all HIV protease inhibitors (eg, ritonavir), and clarithromycin | Azole antifungals (eg, ketoconazole), all HIV protease inhibitors (eg, ritonavir), and clarithromycin | Azole antifungals (eg, ketoconazole), all HIV protease inhibitors (eg, ritonavir), and clarithromycin |
| Drug interactions that decrease drug levels | Rifampin, phenytoin, carbamazepine, and St. John’s wort | Anticonvulsants (eg, phenytoin and carbamazepine), and rifampin | Anticonvulsants (phenytoin and carbamazepine), and rifampin | Anticonvulsants (phenytoin and carbamazepine), and rifampin |
| Laboratory measurement to assess anticoagulant effect† | aPTT, TCT, and dilute TCT | PT/INR and anti–factor Xa assay (for Xa inhibitor) | PT/INR [minimal effect] and anti–factor Xa assay (for Xa inhibitor) | Anti–factor Xa assay (for Xa inhibitor) |
| Available formulations | Capsules, pellets (sprinkles), and oral solution‡ | Tablet and oral solution | Tablet and oral solution | Tablet and oral solution |
| Patient assistance program for drug | Boehringer-Ingelheim | Johnson & Johnson | Bristol Myers Squibb | No program currently available |
aPTT, activated partial thromboplastin time; dTCT, diluted thrombin clotting time; PCC, prothrombin complex concentrates; PT/INR, prothrombin time/international normalized ratio; TCT, thrombin clotting time; TXA, tranexamic acid.
Not approved by the US Food and Drugs Administration for children.
Routine monitoring of anticoagulant effect is not required.
Not approved for infants aged <3 mo.
Periprocedural management of DOACs. ∗Assuming normal renal function and platelet count >50 000 × 109/L; ^surgical bleeding risk stratification has not been studied in children.
Periprocedural management of DOACs. ∗Assuming normal renal function and platelet count >50 000 × 109/L; ^surgical bleeding risk stratification has not been studied in children.
Question 3: what are other considerations pertinent to the case?
All DOACs have shown faster clearance in young infants; therefore, clinicians are required to check the weight and age–based dosing regimens and periodically adjust dose with weight gain (Table 3; Figure 4).9,16,22,36 Furthermore, it is important to determine the oral preparations that are available. For example, dabigatran comes as sprinkles or pellets in various strengths and an oral solution.22 The pellets are dissolved in infant formula or baby foods and given via nasogastric tube or orally but are neither currently available for clinical use nor approved for infants aged <3 months. Rivaroxaban is available as an oral suspension and was favorably assessed for its taste and texture. The half-life of the suspension is shorter (3.24-4.15 hours) than that of tablets (7-17 hours) at doses >10 mg.37
Published rivaroxaban dosing strategy in Einstein Jr and UNIVERSE clinical trials
| Einstein Jr phase 3 (VTE treatment) Body weight–adjusted rivaroxaban regimens in a 20-mg equivalent dose . | UNIVERSE phase 3 (post-Fontan thromboprophylaxis) Body weight–adjusted rivaroxaban regimens in a 10-mg equivalent dose (mg or mL∗) . | ||||
|---|---|---|---|---|---|
| Body weight . | Dose . | Total . | Body weight . | Dose . | Total . |
| 2.6 to <3 kg | 0.8 mg per dose TID | 2.4 mg | 7 to <8 kg | 1.1 mg per dose BID | 2.2 mg |
| 3 to <4 kg | 0.9 mg per dose TID | 2.7 mg | 8 to <10 kg | 1.6 mg per dose BID | 3.2 mg |
| 4 to <5 kg | 1.4 mg per dose TID | 4.2 mg | 10 to <12 kg | 1.7 mg per dose BID | 3.4 mg |
| 5 to <7 kg | 1.6 mg per dose TID | 4.8 mg | 12 to <20 kg | 2 mg per dose BID | 4.0 mg |
| 7 to <8 kg | 1.8 mg per dose TID | 5.4 mg | 20 to <30 kg | 2.5 mg per dose BID | 5.0 mg |
| 8 to <9 kg | 2.4 mg per dose TID | 7.2 mg | |||
| 9 to <10 kg | 2.8 mg per dose TID | 8.4 mg | |||
| 10 to <12 kg | 3 mg per dose TID | 9 mg | |||
| 12 to <30 kg | 5 mg per dose BID | 10 mg | |||
| 30 to <50 kg | 15 mg per dose OD | 15 mg | |||
| ≥50 kg | 20 mg per dose OD | 20 mg | |||
| Einstein Jr phase 3 (VTE treatment) Body weight–adjusted rivaroxaban regimens in a 20-mg equivalent dose . | UNIVERSE phase 3 (post-Fontan thromboprophylaxis) Body weight–adjusted rivaroxaban regimens in a 10-mg equivalent dose (mg or mL∗) . | ||||
|---|---|---|---|---|---|
| Body weight . | Dose . | Total . | Body weight . | Dose . | Total . |
| 2.6 to <3 kg | 0.8 mg per dose TID | 2.4 mg | 7 to <8 kg | 1.1 mg per dose BID | 2.2 mg |
| 3 to <4 kg | 0.9 mg per dose TID | 2.7 mg | 8 to <10 kg | 1.6 mg per dose BID | 3.2 mg |
| 4 to <5 kg | 1.4 mg per dose TID | 4.2 mg | 10 to <12 kg | 1.7 mg per dose BID | 3.4 mg |
| 5 to <7 kg | 1.6 mg per dose TID | 4.8 mg | 12 to <20 kg | 2 mg per dose BID | 4.0 mg |
| 7 to <8 kg | 1.8 mg per dose TID | 5.4 mg | 20 to <30 kg | 2.5 mg per dose BID | 5.0 mg |
| 8 to <9 kg | 2.4 mg per dose TID | 7.2 mg | |||
| 9 to <10 kg | 2.8 mg per dose TID | 8.4 mg | |||
| 10 to <12 kg | 3 mg per dose TID | 9 mg | |||
| 12 to <30 kg | 5 mg per dose BID | 10 mg | |||
| 30 to <50 kg | 15 mg per dose OD | 15 mg | |||
| ≥50 kg | 20 mg per dose OD | 20 mg | |||
Reprinted from McCrindle et al17 (© 2021 by the authors; published on behalf of the American Heart Association, Inc, by Wiley Blackwell), with permission from Wiley Blackwell, and from Male et al16 with permission from Elsevier.
BID, twice daily; OD, once daily; TID, 3 times daily.
Oral suspension 0.1% (1 mg/mL).
Published dabigatran (DIVERSITY-phase 2b/3) dosing strategy according to formulation (VTE treatment).20 Dosing regimen using dabigatran capsules (A), dosing regimen using pellets (B), and dosing regimen using oral liquid formulation (OLF) (C). Adapted from Halton et al20 with permission from Elsevier.
Published dabigatran (DIVERSITY-phase 2b/3) dosing strategy according to formulation (VTE treatment).20 Dosing regimen using dabigatran capsules (A), dosing regimen using pellets (B), and dosing regimen using oral liquid formulation (OLF) (C). Adapted from Halton et al20 with permission from Elsevier.
As the clinician considers a DOAC for this infant, it is important to know how each agent is absorbed via the GI system. Both direct-Xa and -IIa inhibitors are absorbed in the distal part of stomach and proximal small intestine.38,39 Thus, for this infant with a transpyloric tube, a DOAC may lead to suboptimal absorption. Similarly, careful consideration should be given to children with short gut syndrome and the site of absorption of DOACs because most patients lose their jejunum and/or ileum. Additionally, enteral feeds can be influenced by sepsis and other neonatal complications; therefore, brief parenteral anticoagulation may be needed until enteral feeds can resume. Finally, it needs to be recognized that in case of emergent reversal for bleeding, no direct reversal agent has been studied for children nor is it readily available, and a plan should be in place if such bleeding occurs (discussed in “Case 3”).
How did the authors treat this infant?
We adopted a shared clinical decision-making model that included the family and the primary team. The infant was initially treated with LMWH for 2 weeks, during which he remained hemodynamically stable without clinical or radiological worsening of ICH. The family was counseled about the limitations of DOACs for this age group but nonetheless preferred a DOAC. The choice was dictated by access to an infant-friendly liquid formulation. The transpyloric tube was pulled back to function as a nasogastric tube, and the infant was started on the appropriate weight-based dose of rivaroxaban. Because a rivaroxaban-specific anti-Xa assay was unavailable at our institution, a safety plan was put in place for laboratory monitoring with a PT and anti-Xa level calibrated for LMWH, which, although not ideal, can be used as a surrogate marker to assess for presence of the drug in the event of bleeding or worsening of renal function.40 The infant’s PICC was removed 2 weeks after starting rivaroxaban; however, because of persistent occlusive thrombosis at 6 weeks, rivaroxaban was continued to complete treatment for a total of 12 weeks.32 No bleeding occurred, and anticoagulation monitoring was not performed. The authors do not recommend using a DOAC given to preterm infants <37-week postconception age.
Case 2: lower limb DVT in a 4-year-old boy
A 4-year-old boy presented to the emergency department with a right leg limp. A Doppler ultrasound demonstrated an occlusive right superficial femoral vein to popliteal vein thrombosis. There was no preceding history of trauma or other known provoking risk factors. His complete blood count, renal and liver function test results, and coagulation panel were normal.
Question 1: is this patient a candidate for a DOAC therapy?
Although some clinicians may be comfortable with initiating DOAC therapy at diagnosis of a DVT because pediatric formulations are available, we would recommend using initial SOC anticoagulation for this child because of several reasons. First, the phase 3 pediatric DOAC trials required an initial SOC agent (in the Einstein Jr [rivaroxaban] study for 5-9 days and Diversity [dabigatran] study for 5-21 days) before randomization. Although this was to allow for the recruitment in the clinical trials rather than for any pharmacologic or physiologic reasons, there are currently no data in children that suggest that it is safe to primarily initiate treatment with a DOAC. Second, because this is an unprovoked clot, thrombophilia testing for etiology of DVT is warranted, including tests for antiphospholipid antibodies (APLAs) and the lupus anticoagulant before starting a DOAC. It may be prudent to wait for the results of APLAs/lupus anticoagulant because they are currently contraindicated in triple-positive APLA syndrome.41,42
The other important practical question is whether he should be admitted or treated as an outpatient for his DVT. Most pediatric patients require hospitalization for management of an acute DVT for evaluation of the etiology as well as for counseling and education.
After exclusion of anatomic causes of the VTE and negative thrombophilia evaluation results, he was switched to rivaroxaban as an outpatient. He was tolerating rivaroxaban, but the parents wanted to know the duration of anticoagulation and were concerned about bleeding risk and continued activity restrictions.
Question 2: what safety concerns need to be addressed during extended anticoagulation? Can the DOAC intensity be changed from treatment to prophylaxis to reduce the bleeding risk?
Because this is an unprovoked DVT, extending anticoagulation to 6 to 12 merits consideration based on the American College of Chest Physicians and American Society of Hematology guidelines.33,43 However, safety with recreational activities for this young child should be included in the discussion with the family. Brief interruption of anticoagulation during activities at high risk of bleeding (eg, trampoline party) is reasonable to maintain a good quality of life, although this has not been formally studied. Additionally, plan for management of bleeding on a DOAC agent should be in place (discussed in “Case 3”). The other consideration is reducing treatment dose to prophylactic intent after 6 months. It is important to note that both the Diversity and Einstein Jr studies continued treatment for secondary prevention of VTE in children beyond the initial 3-month treatment phase, but the dosing regimen was not changed.23 The UNIVERSE study used a body weight–adjusted rivaroxaban regimen in a 10 mg equivalent dose as post-Fontan prophylaxis for children with hypoplastic left heart syndrome as opposed to the 20 mg equivalent in the VTE studies.17 One could consider lowering the dose in a similar ratio to maintain the quality of life, although there are no data to support this. One could also consider continuing treatment dosing, given the low bleeding rate in clinical trials and the available data on efficacy.16,22,23
Question 3: what are other considerations pertinent to this patient?
This is a young child, and long-term safety (beyond 1 year) of DOACs in children has not been studied. For example, the negative impact of extended anticoagulation with vitamin K antagonists on bone density in children has been reported, and screening for osteoporosis is recommended.44 Similarly, the adverse effects of UFH on angiogenesis, bone remodeling, and osteoporosis are known.45-47 In adult studies, early data suggest minimal impact of DOAC on bone health.48 Bone density was measured in the SAXOPHONE study, but results were not reported.20 Additional data on off-target effects of DOACs in children on extended anticoagulation, especially on growth and development, and bone density, are warranted (Table 4).
Key age and disease specific management issues and areas of future study of DOACs
| Special population . | Clinical challenges and future research needs . |
|---|---|
| Age groups | |
| Neonates |
|
| Toddler/young child |
|
| Teenager |
|
| Medical comorbidities | |
| Cancer |
|
| Renal/liver dysfunction, GI malabsorption, or short gut |
|
| Special population . | Clinical challenges and future research needs . |
|---|---|
| Age groups | |
| Neonates |
|
| Toddler/young child |
|
| Teenager |
|
| Medical comorbidities | |
| Cancer |
|
| Renal/liver dysfunction, GI malabsorption, or short gut |
|
PD, pharmacodynamic.
How did the authors treat this patient?
This patient was treated with LMWH for 2 weeks until the results of thrombophilia testing were available. Selection of a direct-Xa or -IIa inhibitor was based on the availability of a pediatric formulation, family’s dosing preference, physician’s comfort, and patient's insurance coverage. He was started on rivaroxaban suspension after careful discussion with the family about safety precautions. His 6-month follow-up Doppler ultrasound showed chronic occlusion in the superficial femoral vein with collaterals, resolution of his popliteal vein thrombus, and evidence of mild postthrombotic syndrome. He was continued on therapeutic anticoagulation for a total of 12 months and did not develop any bleeding episode or a recurrence. His follow-up imaging at 12 months continued to show chronic occlusion of the superficial femoral vein but no new thrombosis and normal VTE biomarkers.49 Anticoagulation was discontinued because of lifestyle limitations and family preference with close clinical follow-up.
Case 3: cerebral sinus venous thrombosis in a child with cancer
A 12-year-old boy with morbid obesity (weight, 100 kg; body mass index [BMI], 36 kg/m2) with a diagnosis of high-risk acute lymphoblastic leukemia (ALL) in the delayed intensification phase of chemotherapy developed a seizure secondary to an extensive superior sagittal sinus thrombosis. Magnetic resonance imaging showed scattered cerebral infarcts with evidence of raised intracranial pressure but no midline shift. He had received asparaginase therapy, and his antithrombin (AT) activity was low at 30% (normal range, 80%-135%). He had a phobia of needles; therefore, LMWH was not preferred by the family.
Question 1: is this patient a candidate for DOAC therapy?
What is the evidence that DOACs are effective anticoagulants for thrombosis treatment in children with ALL and/or cerebral sinovenous thrombosis (CSVT)? First, in vitro studies of plasma samples of patients with ALL have shown that direct thrombin inhibitors provide a consistent anticoagulant response measured by the reduction in endogenous thrombin generation compared with LMWH and that this effect is independent of AT activity.50 Second, in the phase 2b/3 Diversity and Einstein Jr clinical trials, 11% and 20% of children, respectively, were treated for CSVT with comparable efficacy compared with the SOC treatment.16,22,51 These data support considering a DOAC for this patient; however, one needs to determine whether it is safe to commence with a DOAC immediately or after initial treatment with an SOC option.
Question 2: what safety concerns require a priori attention before initiating DOACs?
This patient has an extensive CSVT with the presence of ischemic and hemorrhagic infarcts, suggesting secondary venous hypertension and an increased bleeding risk.52 Therefore, the immediate safety concern is the presence of ICH due to intracranial hypertension. Additionally, this patient is at ongoing risk of chemotherapy-related side effects, including thrombocytopenia, mucositis, neutropenia, sepsis, the need for invasive procedures, the use of nephrotoxic and hepatotoxic chemotherapeutic agents, and concomitant use of CYP3A4 and/or P-gp agents leading to delayed or faster clearance of the DOACs. Therefore, the clinician should anticipate these challenges, and if recommending DOACs, it is of utmost importance that a specific therapeutic monitoring plan and emergency reversal plan be in place. Although a platelet count threshold is not established for patients on anticoagulants, the clinical trials required a platelet count > 50 × 109/L for treatment and > 20 × 109/L for prophylactic anticoagulation.16,18,22
For patients with active bleeding or undergoing invasive procedures, one should consider keeping the platelet count > 50 × 109/L and maintaining it at that level for at least 24 hours. The risk of GI or mucosal bleeding is higher for patients with cancer receiving chemotherapy because of mucositis and require close monitoring. Patients receiving asparaginase therapy can also develop liver synthetic dysfunction resulting in hypoalbuminemia and coagulopathy, including acquired AT deficiency. Because the majority of DOACs (except edoxaban) are highly protein bound (>85%), hypoalbuminemia may alter the anticoagulant effect by increasing free drug but may also lead to faster clearance (Table 2). However, the true anticoagulant effect of DOACs in patients with low albumin is unclear. Additionally, one may consider extending anticoagulant treatment until after the completion of asparaginase therapy or its re-exposure for secondary thromboprophylaxis.53,54
For procedures such as lumbar puncture, which are considered high risk of bleeding, it is safer to hold therapeutic anticoagulation for at least 48 hours before and 24 hours after the procedure, provided the patient’s renal and liver functions are normal (Figure 3).55-58 If there is bleeding or a need for emergency reversal, readily available, in-house, special coagulation assays could be performed with an intent to assess the presence or absence of drug though they may not correlate with the precise drug level.40,59 Clinicians are advised to check with their special coagulation laboratory regarding access to DOAC monitoring assays (Table 2).
In this case, obesity needs additional discussion, because it can affect drug PK by increasing the volume of distribution and enhancing drug clearance and PK/pharmacodynamics effects of other concomitant drugs. Patients with obesity (BMI > 30 kg/m2) were underrepresented in the phase 3 clinical trials (a few children weighing up to 137 kg were included in the Einstein Jr trial, but normal BMI was required in the Diversity study).16,22 Available literature on adult patients shows that the BMI has either a modest or no effect on DOAC concentration and anti-Xa levels and comparable efficacy and safety to that for patients with normal weight and therefore do not require a dose adjustment.60-63 However, recent adult guidelines suggest using apixaban or rivaroxaban for patients with BMI >40 kg/m2.64 Because there are practically no data about using DOACs for children who are overweight and have obesity, it may be reasonable to extrapolate adult-based guidelines to this subgroup.
Question 3: what are other considerations pertinent to this patient?
This patient has ischemic cerebral infarcts and therefore is at risk of ischemic areas converting into hemorrhagic infarcts especially in the presence of raised intracranial pressure. Given the limited experience of treating CSVT with DOAC (∼100 children so far), it is safer to start treatment with an SOC anticoagulant before commencing with DOAC therapy. Emergency reversal of a DOAC in the event of major bleeding poses a major challenge for children because of the limited access to a reversal agent. Although idarucizumab and andexanet-α are approved by the US Food and Drugs Administration for adults for reversal of dabigatran and rivaroxaban/apixaban, respectively, it is neither approved nor tested for children.65-67 There are only 2 published case reports of using idarucizumab and andexanet-α for children, for overdose and bleeding reversal.68,69 Interestingly, a recent survey of pediatric hematologists assessing preferences for reversal of bleeding associated with DOACs reported prothrombin complex concentrates as the preferred option.70 In case of a DOAC overdose within 2 to 4 hours, gastric lavage with activated charcoal is suggested. For mild bleeding symptoms, we recommend using topical therapy and supportive interventions such as pressure or nasal packing. For moderate and severe bleeding, including life-threatening bleeding, holding anticoagulation, volume resuscitation, and/or blood product support along with reversal with 3 or 4 factor prothrombin complex concentrates71 and/or a DOAC specific reversal agent should be considered.6,65,68,72,73 Further discussion of reversal agents is beyond the scope of this article, and readers are advised to read recently published review articles.28,74,75
How did authors treat this patient?
Because there was a concern for worsening of ICH, the patient was commenced on IV argatroban because its anticoagulant effect is independent of AT and it has short duration of action as well as an accumulating experience of its use for children.76 The patient was switched to oral dabigatran at 330 mg twice daily immediately after discontinuation of argatroban. Although he is an adolescent with BMI comparable with that of an adult, we recommend treatment with age and weight–based pediatric nomogram. Dabigatran was chosen because of its direct thrombin effect and availability of a pediatric formulation and idaricizumab, if needed, for severe bleeding.72,73,77-80 The family and primary team were educated about the limitations of therapeutic drug monitoring and lack of available data on using idaricizumab for children. Furthermore, an emergency monitoring (thrombin or eccarin clotting time) and reversal plan (idaricizumab) was placed in the electronic health record, and the importance of holding anticoagulation before procedures (Figure 2) was reviewed with the family and the primary oncologist. The patient remained on dabigatran for 6 months without bleeding complications and had complete resolution of the CSVT.
Case 4: a teenager with a lower extremity DVT while on a combined OCP and inherited thrombophilia
A 16-year-old female weighing 46 kg was prescribed a combined estrogen/progesterone oral contraceptive pill (OCP) because of heavy menstrual bleeding (HMB). A comprehensive evaluation for bleeding disorders showed negative results. After 2 months, she developed right leg swelling and was diagnosed with an ilio-femoral DVT. Of note, there was a strong family history of DVT/PE. As a result, a thrombophilia evaluation was undertaken, and the patient was found to be compound-heterozygous for factor V Leiden and the prothrombin gene mutations. She was otherwise healthy with no other medical problems. She participated in cheerleading and was anxious to know the duration of anticoagulation and whether she would be able to participate.
Question 1: is this patient a candidate for a DOAC agent at the outset?
Certainly, she appears to be an excellent candidate for a DOAC; however, can she be initiated on it without the requisite 5 days of SOC anticoagulation? As previously mentioned, this “wash-in” period in the clinical trials (and hence prescribing information) was for the purposes of study design logistics. The trickier issue is what to do with the OCPs and how to manage the HMB that led to their use especially considering that this bleeding could be exacerbated by DOACs.81,82 Although a detailed discussion of these issues is beyond the scope of this article, one should consider an alternative method for managing HMB that has a lower risk of VTE, such as an intrauterine device.
Question 2: what safety concerns need to be addressed?
Studies have indicated that DOACs may worsen menstrual bleeding in women of childbearing age.82,83 Therefore, menstrual blood loss should be carefully monitored using one of the available validated scoring systems or apps,84-87 with clear instructions to report back if the amount of bleeding exceeds a certain threshold.
Question 3: what are other considerations pertinent to this patient?
The other major consideration for this patient is the potential need for extended anticoagulation because of compound thrombophilia and a strong family history of thrombosis. There are no published studies of children using secondary thromboprophylaxis with DOACs beyond 15 months.8,23 Furthermore, after 6 months, one could consider lowering the DOAC dose in a similar ratio to that for adults,88 however, there are no data to support this plan.
Another consideration, especially for teenagers, should include sports participation. Contact sports are generally not recommended while a patient is on full-intensity anticoagulation. We suggest using the sports stratification proposed by the National Hemophilia Foundation to counsel families regarding safe sports participation.89 This patient is a cheerleader and careful thought should be given to returning to cheerleading because prohibiting her from participation can have significant impact on mental health. For extended anticoagulation, the issue of bone health with long-term DOAC therapy merits consideration, but preclinical data suggest that they do not cause osteoporosis.90-92 Finally, a discussion on contraception to prevent pregnancy on a DOAC should be undertaken for all young women of childbearing potential, because pregnant women were excluded from both the pediatric and adult DOAC VTE treatment trials.
How did the authors treat this patient?
The patient was admitted to the hospital for evaluation and discussion of treatment options after 1 dose of fondaparinux in the emergency room. The OCPs were discontinued. Given the published data on efficacy and safety in pediatrics, both rivaroxaban and dabigatran would be reasonable options for a DOAC. Because the patient’s hemoglobin level was normal and the patient, her parents, and the treating physician were comfortable with her being discharged, she was started on rivaroxaban 15 mg once daily the next day and sent home with clear instructions regarding monitoring the HMB. It is important to note that in the Einstein Jr trial, the dose studied was designed to achieve the same area under the dissolution curve in pediatrics compared with the 20 mg once daily dose in adults (the initial loading dose of 15 mg twice daily in adults was never investigated). We recommend that a pediatric patient should be started per the pediatric dose nomogram. Because she continued to experience HMB, albeit not worsened by rivaroxaban, after 2 months (and 2 period cycles), a levonorgestrel-containing intrauterine device was placed.
The final issue in this case is her double thrombophilia and the family history of thrombosis. There are no studies that can address this issue with respect to long-term anticoagulation resulting in challenges in decision-making.8,23 For this patient (and similar ones), we suggest continuing anticoagulation indefinitely and reevaluating the need to continue anticoagulation annually. So long as the patient is not experiencing bleeding side effects, it is reasonable to continue anticoagulation indefinitely given the potential benefit of preventing a VTE (the next one could be life-threatening pulmonary embolism) vs the risk of serious bleeding.
Conclusion and future directions
DOACs have, by far, been the most studied anticoagulants in children. They offer an oral alternative for treatment and secondary prevention of thrombosis for children of all ages and are increasingly being used in clinical practice beyond the pediatric indications studied in the recently published clinical trials. Nonetheless, the choice of DOACs depends upon the available formulation and other patient-specific factors. Although multiple DOACs may be available soon, at an institutional level, one could consider choosing a particular DOAC consistently to build experience and consistency in practice for the involved team, including nurses, pharmacists, and other staff. Currently, DOACs are unsuitable or contraindicated for children with mechanical valves, triple-positive APLA syndrome, severe liver or kidney diseases, those with limited GI absorption, or those on concomitant medications that are inducers and inhibitors of CYP3A4 and P-gp. Considering the rarity of VTE in pediatrics, it is unlikely that clinical trials will be performed in each specific population. Therefore, data collection on real-world use of DOACs through large national and international networks such as the American Thrombosis and Hemostasis Network93 and International Pediatric Thrombosis Network94 is critical to assess benefit-risk profile. Areas of future study should include evaluation of reversal agents for DOACs, use in special populations, the ability to monitor compliance, and their off-target effects (Table 4). While the pediatric hematologist gains more experience using DOACs, we recommend using collective knowledge and a shared decision-making model involving discussion with patients and families to ensure safety and efficacy.
Acknowledgments
R.V.B. dedicates this article to Paul Monagle, who inspired her to pursue a career in pediatric thrombosis, and to mentors Alexis Thompson and Robert Liem, who guided her career development.
Authorship
Contribution: R.V.B. developed the educational objectives and identified gaps in DOAC management; and all authors contributed equally to the writing, editing, and approval of the final submitted manuscript version.
Conflict-of-interest disclosure: R.V.B. reports being an institutional principal investigator for pediatric VTE trials funded by Pfizer and Bayer and receiving an investigator-initiated grant from the Bristol Myers Squibb–Pfizer alliance to study risk factors of neonatal thrombosis. G.Y. was an institutional principal investigator for pediatric VTE trials funded by Bayer and Daiichi-Sankyo; has received consulting fees and honoraria from Bayer and Daiichi-Sankyo; and received a licensing fee from Viatris. A.A.S. was an institutional principal investigator for pediatric VTE trials funded by the National Institutes of Health, Pfizer, and Boehringer-Ingelheim.
Correspondence: Rukhmi V. Bhat, Center for Cancer and Blood Disorders, Ann and Robert H. Lurie Children’s Hospital of Chicago, Northwestern University Feinberg School of Medicine, 225 E Chicago, Chicago, IL 60611; email: rbhat@luriechildrens.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal