Pivotal TRANSCEND NHL 001 study demonstrated the efficacy and safety of liso-cel as third-line or later treatment for patients with R/R LBCL.
With 2-year follow-up, liso-cel showed high response rates, durable remissions, and a manageable safety profile for patients with R/R LBCL.
Visual Abstract
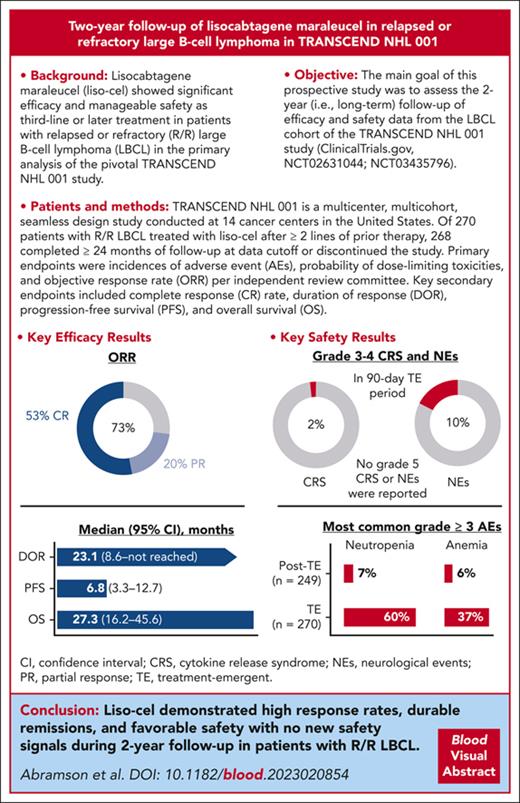
Lisocabtagene maraleucel (liso-cel) demonstrated significant efficacy with a manageable safety profile as third-line or later treatment for patients with relapsed or refractory (R/R) large B-cell lymphoma (LBCL) in the TRANSCEND NHL 001 study. Primary end points were adverse events (AEs), dose-limiting toxicities, and objective response rate (ORR) per independent review committee. Key secondary end points were complete response (CR) rate, duration of response (DOR), progression-free survival (PFS), and overall survival (OS). After 2-year follow-up, patients could enroll in a separate study assessing long-term (≤15 years) safety and OS. Liso-cel–treated patients (N = 270) had a median age of 63 years (range, 18-86 years) and a median of 3 prior lines (range, 1-8) of systemic therapy, and 181 of them (67%) had chemotherapy-refractory LBCL. Median follow-up was 19.9 months. In efficacy-evaluable patients (N = 257), the ORR was 73% and CR rate was 53%. The median (95% confidence interval) DOR, PFS, and OS were 23.1 (8.6 to not reached), 6.8 (3.3-12.7), and 27.3 months (16.2-45.6), respectively. Estimated 2-year DOR, PFS, and OS rates were 49.5%, 40.6%, and 50.5%, respectively. In the 90-day treatment-emergent period (N = 270), grade 3 to 4 cytokine release syndrome and neurological events occurred in 2% and 10% of patients, respectively. The most common grade ≥3 AEs in treatment-emergent and posttreatment-emergent periods, respectively, were neutropenia (60% and 7%) and anemia (37% and 6%). Liso-cel demonstrated durable remissions and a manageable safety profile with no new safety signals during the 2-year follow-up in patients with R/R LBCL. These trials were registered at www.ClinicalTrials.gov as #NCT02631044 and #NCT03435796.
Introduction
Patients with relapsed or refractory (R/R) large B-cell lymphoma (LBCL) for whom standard second-line therapy has failed have poor outcomes using conventional therapies, with very few patients achieving complete response (CR) and virtually no patients achieving long-term remission.1,2 Outcomes have been particularly poor for patients with chemotherapy-refractory disease, with CR rates of ∼7% to conventional therapy and an overall survival (OS) of 6 months.3 In addition, older age (>65 years), central nervous system (CNS) involvement,4,5 and presence of medical comorbidities6 have been associated with poor outcomes.
Autologous chimeric antigen receptor (CAR) T-cell therapies have demonstrated significant clinical benefits for several hematologic malignancies, including R/R LBCL, providing a valuable treatment option for these patients.7-12 Lisocabtagene maraleucel (liso-cel) is an autologous, CD19-directed, 4-1BB CAR T-cell product composed of CD8+ and CD4+ CAR+ T cells.10 The consistent total and relative doses of CD8+ and CD4+ CAR+ T cells and low variability in CD8+:CD4+ ratio could potentially influence the incidence and severity of cytokine release syndrome (CRS) and neurological events (NEs).8,13-16
In the primary analysis of the multicenter, seamless design TRANSCEND NHL 001 (TRANSCEND [NCT02631044]) study, liso-cel demonstrated substantial efficacy in patients with R/R LBCL, with an objective response rate (ORR) of 73%, CR rate of 53%, a median duration of response (DOR) not reached (NR; 95% confidence interval [CI], 8.6-NR) at median follow-up for DOR of 12.0 months (95% CI, 11.2-16.7), and 1-year estimated DOR rate of 55% (but 65% among patients who achieved CR).10 Estimated 1-year progression-free survival (PFS) and OS rates were 44% and 58%, respectively. Liso-cel was associated with low incidences of grade 3 to 4 CRS (2%) and NEs (10%), with no grade 5 CRS or NEs reported. Here, we present 2-year follow-up data from the LBCL cohort of TRANSCEND.
Methods
Study design and participants
TRANSCEND (NCT02631044) is a multicenter, multicohort, seamless design study conducted at 14 cancer centers in the United States. The study design, including full eligibility criteria, has been previously reported.10 In brief, eligible patients in the LBCL cohort were aged ≥18 years and had measurable disease, as assessed by positron emission tomography (PET) positivity. Eligible histologies included R/R diffuse LBCL (de novo or transformed from any indolent lymphoma), high-grade B-cell lymphoma with rearrangements in MYC and either BCL2, BCL6, or both (double-hit or triple-hit lymphoma), primary mediastinal B-cell lymphoma, or follicular lymphoma grade 3B.17 Patients must have received ≥2 lines of prior systemic therapy (including an anthracycline and an anti-CD20–targeted agent) with subsequent progression; previous autologous or allogeneic hematopoietic stem cell transplantation was allowed. Patients with moderate medical comorbidities and secondary CNS lymphoma were allowed. No minimum absolute lymphocyte count was required for enrollment.
All patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization Good Clinical Practice guidelines, and applicable regulatory requirements. The study protocol and amendments were approved by institutional review boards at participating sites.
Procedures
Full study procedures have been described.10 In brief, CD8+ and CD4+ T cells were immunomagnetically selected from patients’ leukapheresis material, then independently activated, transduced, and expanded to manufacture liso-cel. Bridging therapy for disease control was allowed at clinician discretion, but reconfirmation of PET-positive disease was required before lymphodepletion with 30 mg/m2 fludarabine and 300 mg/m2 cyclophosphamide IV daily for 3 days. Liso-cel was administered from 2 to 7 days after lymphodepleting chemotherapy as 2 sequential infusions of CD8+ and CD4+ CAR+ T cells at 1 of 3 target doses of 50 × 106, 100 × 106, or 150 × 106 CAR+ T cells. The seamless design principle was used.18,19 The study consisted of dose-finding, dose-expansion, and dose-confirmation phases.10 All dose levels were assessed for dose-limiting toxicities and clinical activity based on CR rates in dose-finding and dose-expansion phases. The recommended regimen of 100 × 106 CAR+ T cells was tested during dose confirmation. Patients who achieved a CR after liso-cel infusion and subsequently had progressive disease could receive retreatment with liso-cel. Outpatient administration of liso-cel was allowed at the investigator’s discretion.
Response was evaluated by PET/computed tomography per Lugano 2014 criteria,20 assessed by independent review committee (IRC). Adverse events (AEs), including NEs (investigator-identified neurological AEs related to liso-cel), were graded using the National Cancer Institute Common Terminology Criteria for AEs, version 4.03, except for CRS, which was graded using the Lee 2014 criteria.21,22 Prolonged cytopenia was defined as any grade ≥3 laboratory result of decreased hemoglobin (Hb), neutrophil count, or platelet count on the day 29 visit. The treatment-emergent period was defined as the time from initiation of liso-cel administration up to and including 90 days after liso-cel infusion. Any AEs occurring after the initiation of another anticancer therapy or liso-cel retreatment were not considered treatment-emergent AEs. The posttreatment-emergent period was defined as starting from 91 days after liso-cel infusion or from initiation of another anticancer therapy or liso-cel retreatment (if patients initiated another anticancer therapy or liso-cel retreatment before 91 days after liso-cel infusion). Additional information about study design, treatment (including lymphodepleting chemotherapy and bridging therapy), safety assessments, and cellular kinetics has been published.10,23,24
Outcomes
The primary end points were incidence of AEs, probability of dose-limiting toxicities, and ORR, defined as the proportion of patients who achieved a CR or partial response (PR) as assessed by IRC per Lugano 2014 criteria.20 Key secondary end points included CR rate; DOR, defined as the time from first response to progressive disease or death; PFS, defined as the time from first infusion of liso-cel to progressive disease or death; OS, defined as the time from liso-cel infusion to death from any cause; and cellular kinetics. Liso-cel transgene levels were assessed over time in peripheral blood by quantitative polymerase chain reaction.23,24
Long-term follow-up procedures
Patients were followed for 2 years after the last dose of liso-cel to assess safety and efficacy in TRANSCEND. All patients who completed the 2-year follow-up period or who withdrew from the study after receiving ≥1 dose of liso-cel were asked to enroll in a separate long-term follow-up study (NCT03435796). Safety and OS in the long-term follow-up study will be assessed for up to 15 years after liso-cel infusion; however, no IRC response assessments will be performed. Safety and OS analyses included data from patients who completed TRANSCEND and enrolled in the subsequent long-term follow-up study. Analyses by IRC of ORR, CR rate, DOR, and PFS only included data from TRANSCEND. Cellular kinetics in the long-term follow-up study were monitored by droplet digital polymerase chain reaction.25
Statistical analysis
Data across all dose levels were combined because of the absence of a clear dose-related toxicity relationship and a lack of efficacy difference between individual dose levels.10 Safety analyses included all patients who received ≥1 dose of liso-cel (liso-cel–treated set). Efficacy analyses included all patients with confirmed PET-positive disease per IRC assessment before receiving ≥1 dose of liso-cel (efficacy-evaluable set). Cellular kinetics were assessed for all patients who received liso-cel who had baseline and on-study cellular kinetic measurements. The 1-sided significance level for the primary efficacy analysis was 0.021 for the testing of the null hypotheses of ORR of ≤40% and CR rate of ≤20%, respectively; results from the primary efficacy analysis testing have been previously published10 and were not repeated. Kaplan-Meier methodology was used to estimate median and 6-, 12-, 18-, and 24-month rates with 95% CIs for DOR, PFS, and OS; follow-up time and 95% CIs were calculated using the reverse Kaplan-Meier method. Patients without DOR or PFS events were censored at the date of the last adequate disease assessment on or before the earliest censoring event. Censoring events included loss of follow-up, study discontinuation or completion, receipt of another anticancer treatment (including retreatment with liso-cel), receipt of transplant, and ≥2 consecutive missed scheduled disease assessments. For OS assessment, data from surviving patients were censored at the last time the patient was known to be alive.
Results
Patients and treatment
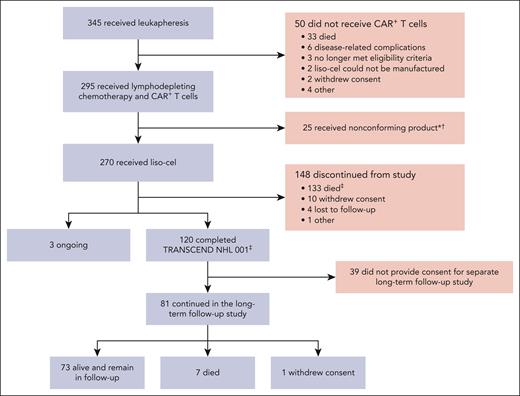
Between 11 January 2016 and 24 September 2019, a total of 345 patients in the LBCL cohort had leukapheresis, with 295 patients receiving CAR+ T cells (Figure 1): 270 received liso-cel (liso-cel–treated set) and 25 received a nonconforming CAR+ T-cell product (ie, one of the CD8 or CD4 cell components did not meet one of the requirements to be considered liso-cel but was considered appropriate for infusion). Efficacy and safety outcomes at the 2-year follow-up among the patients who received nonconforming product were consistent with previously reported data.10 Seventeen patients in the liso-cel–treated set received a subsequent infusion of liso-cel after disease progression following a best response of CR.
Flow of patients through the study (CONSORT diagram). ∗Denotes that nonconforming product is defined as any product wherein one of the CD8 or CD4 cell components did not meet one of the requirements to be considered liso-cel but was considered appropriate for infusion. †Denotes that 7 patients among those who received nonconforming product in TRANSCEND continued in the long-term follow-up study. ‡Denotes that 1 patient who died after study completion was included among deaths occurring in the posttreatment-emergent period.
Flow of patients through the study (CONSORT diagram). ∗Denotes that nonconforming product is defined as any product wherein one of the CD8 or CD4 cell components did not meet one of the requirements to be considered liso-cel but was considered appropriate for infusion. †Denotes that 7 patients among those who received nonconforming product in TRANSCEND continued in the long-term follow-up study. ‡Denotes that 1 patient who died after study completion was included among deaths occurring in the posttreatment-emergent period.
As of the data cutoff date (4 January 2021), the TRANSCEND study was ongoing. In the liso-cel–treated set (N = 270), 268 patients had ≥24 months of follow-up (ie, completed the TRANSCEND study) or discontinued the study; 2 patients who were ongoing treatment in TRANSCEND at the data cutoff had <24 months of follow-up. Overall, 120 patients completed the 2-year TRANSCEND study period, and 81 of these patients enrolled in the separate long-term follow-up study of safety and OS for up to 15 years. The combined overall median follow-up for the liso-cel–treated set in TRANSCEND and the long-term follow-up study was 19.9 months (range, 0.2-55.6). The combined total on-study follow-up time was 429 patient-years.
Patient demographics and baseline disease characteristics for the liso-cel–treated set are shown in Table 1. The median age was 63 years (range, 18-86 years); 112 (41%) patients were aged ≥65 years. The most common histologic subtypes were diffuse LBCL not otherwise specified (n = 137 [51%]); diffuse LBCL transformed from indolent lymphoma (n = 78 [29%], which included that transformed from follicular lymphoma (n = 60 [22%]) and that transformed from other indolent non-Hodgkin lymphoma subtypes (n = 18 [7%]); and high-grade B-cell lymphoma with rearrangements of MYC and BCL2, BCL6, or both (n = 36 [13%]). Patients were heavily pretreated, with a median of 3 prior lines (range, 1-8) of systemic therapy: 181 (67%) had chemotherapy-refractory disease and 119 (44%) had never achieved a CR with prior therapy. Seven (3%) patients had secondary CNS lymphoma. A total of 159 (59%) patients received bridging therapy before lymphodepleting chemotherapy and CAR T-cell infusion.
Demographics and disease characteristics
| . | Liso-cel–treated set (N = 270) . |
|---|---|
| Sex, n (%) | |
| Male | 174 (64) |
| Female | 96 (36) |
| Median age (range), y | 63 (18-86) |
| ≥65 y | 112 (41) |
| ≥75 y | 27 (10) |
| Ethnicity, n (%) | |
| Not Hispanic or Latino | 233 (86) |
| Hispanic or Latino | 27 (10) |
| Not reported | 10 (4) |
| Race, n (%) | |
| White | 233 (86) |
| Black or African American | 12 (4) |
| Asian | 11 (4) |
| American Indian or Alaska Native | 2 (<1) |
| Multiple | 1 (<1) |
| Not reported | 11 (4) |
| Non-Hodgkin lymphoma subtypes, n (%) | |
| Diffuse LBCL, not otherwise specified | 137 (51) |
| Diffuse LBCL transformed from indolent lymphoma | 78 (29) |
| Transformed from follicular lymphoma | 60 (22) |
| Transformed from other indolent non-Hodgkin lymphoma subtypes | 18 (7) |
| High-grade B-cell lymphoma with gene rearrangements in MYC and either BCL2, BCL6, or both | 36 (13) |
| Primary mediastinal B-cell lymphoma | 15 (6) |
| Follicular lymphoma grade 3B | 4 (1) |
| ECOG PS at screening, n (%) | |
| 0 | 111 (41) |
| 1 | 155 (57) |
| 2 | 4 (1) |
| Before lymphodepleting chemotherapy | |
| Median sum of the product of perpendicular diameters (range), cm2 | 22.5 (0.8-418.6) |
| Sum of the product of perpendicular diameters ≥50 cm2, n (%) | 73 (28) |
| Median lactate dehydrogenase (range), U/L | 264.5 (112-11 933) |
| Lactate dehydrogenase ≥500 U/L, n (%) | 58 (21) |
| Creatinine clearance <60 mL/min, n (%) | 51 (19) |
| Median baseline C-reactive protein (range), mg/L | 27.4 (0.25-2158.0) |
| Left ventricular ejection fraction ≥40% and <50%, n (%) | 13 (5) |
| Median previous lines of systemic therapy (range) | 3 (1-8) |
| Number of previous lines of systemic therapy,∗n (%) | |
| 1 | 9 (3) |
| 2 | 122 (45) |
| 3 | 68 (25) |
| ≥4 | 71 (26) |
| Chemotherapy refractory,† n (%) | 181 (67) |
| Received previous HSCT, n (%) | 94 (35) |
| Autologous HSCT | 90 (33) |
| Allogeneic HSCT | 9 (3) |
| Never achieved CR with previous therapy, n (%) | 119 (44) |
| Secondary CNS lymphoma, n (%) | 7 (3) |
| Received bridging therapy, n (%) | 159 (59) |
| . | Liso-cel–treated set (N = 270) . |
|---|---|
| Sex, n (%) | |
| Male | 174 (64) |
| Female | 96 (36) |
| Median age (range), y | 63 (18-86) |
| ≥65 y | 112 (41) |
| ≥75 y | 27 (10) |
| Ethnicity, n (%) | |
| Not Hispanic or Latino | 233 (86) |
| Hispanic or Latino | 27 (10) |
| Not reported | 10 (4) |
| Race, n (%) | |
| White | 233 (86) |
| Black or African American | 12 (4) |
| Asian | 11 (4) |
| American Indian or Alaska Native | 2 (<1) |
| Multiple | 1 (<1) |
| Not reported | 11 (4) |
| Non-Hodgkin lymphoma subtypes, n (%) | |
| Diffuse LBCL, not otherwise specified | 137 (51) |
| Diffuse LBCL transformed from indolent lymphoma | 78 (29) |
| Transformed from follicular lymphoma | 60 (22) |
| Transformed from other indolent non-Hodgkin lymphoma subtypes | 18 (7) |
| High-grade B-cell lymphoma with gene rearrangements in MYC and either BCL2, BCL6, or both | 36 (13) |
| Primary mediastinal B-cell lymphoma | 15 (6) |
| Follicular lymphoma grade 3B | 4 (1) |
| ECOG PS at screening, n (%) | |
| 0 | 111 (41) |
| 1 | 155 (57) |
| 2 | 4 (1) |
| Before lymphodepleting chemotherapy | |
| Median sum of the product of perpendicular diameters (range), cm2 | 22.5 (0.8-418.6) |
| Sum of the product of perpendicular diameters ≥50 cm2, n (%) | 73 (28) |
| Median lactate dehydrogenase (range), U/L | 264.5 (112-11 933) |
| Lactate dehydrogenase ≥500 U/L, n (%) | 58 (21) |
| Creatinine clearance <60 mL/min, n (%) | 51 (19) |
| Median baseline C-reactive protein (range), mg/L | 27.4 (0.25-2158.0) |
| Left ventricular ejection fraction ≥40% and <50%, n (%) | 13 (5) |
| Median previous lines of systemic therapy (range) | 3 (1-8) |
| Number of previous lines of systemic therapy,∗n (%) | |
| 1 | 9 (3) |
| 2 | 122 (45) |
| 3 | 68 (25) |
| ≥4 | 71 (26) |
| Chemotherapy refractory,† n (%) | 181 (67) |
| Received previous HSCT, n (%) | 94 (35) |
| Autologous HSCT | 90 (33) |
| Allogeneic HSCT | 9 (3) |
| Never achieved CR with previous therapy, n (%) | 119 (44) |
| Secondary CNS lymphoma, n (%) | 7 (3) |
| Received bridging therapy, n (%) | 159 (59) |
ECOG PS, Eastern Cooperative Oncology Group performance status; HSCT, hematopoietic stem cell transplantation.
The original study protocol enrolled patients with ≥2 previous lines of treatment, including 9 patients who had received 1 line of systemic treatment plus consolidation with HSCT or radiation. The protocol was amended to require at least 2 previous lines of systemic treatment.
The status was chemotherapy refractory if the patient achieved stable disease or progressive disease to the last chemotherapy-containing regimen or relapsed <12 months after autologous stem cell transplantation; otherwise, the status was chemotherapy sensitive.
Efficacy
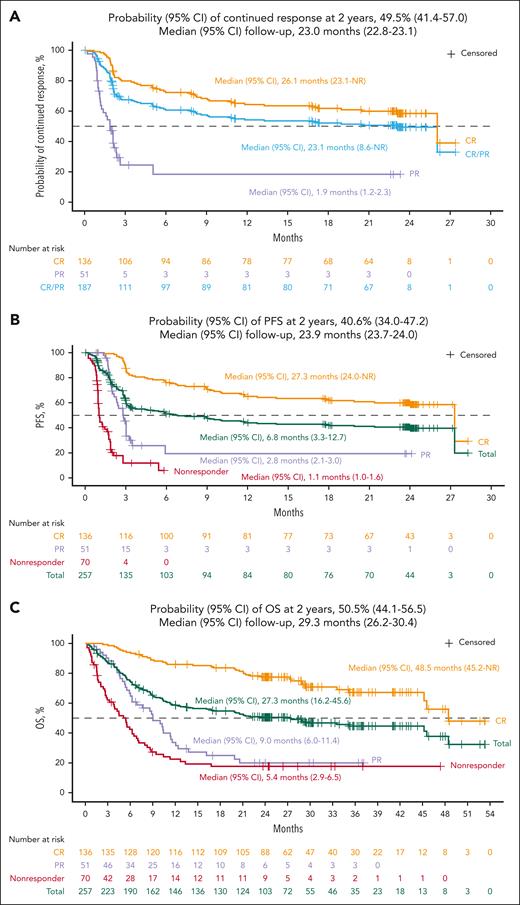
In the efficacy-evaluable set (N = 257), the IRC-assessed ORR was 73% (n = 187; 95% CI, 66.9-78.1; Table 2), with 53% of patients achieving a best response of CR (n = 136; 95% CI, 46.6-59.2). The median time to first CR or PR was 0.95 months (range, 0.7-8.9), and the median time to CR was 0.95 months (range, 0.8-12.5). The median DOR was 23.1 months (95% CI, 8.6-NR) with a median follow-up of 23.0 months (95% CI, 22.8-23.1). The probability of continued response at 24 months was 49.5% (95% CI, 41.4-57.0). The median DOR among patients with a best response of CR was 26.1 months (95% CI, 23.1-NR; Figure 2A). The patient with an event at 26 months died of sepsis while in an ongoing CR at the last evaluation. No patients relapsed later than 23 months from the time of their first response in TRANSCEND (Figure 2A).
Summary of efficacy end points in the efficacy-evaluable set
| . | Efficacy-evaluable set (N = 257) . |
|---|---|
| ORR,∗ n (%, 95% CI†) | 187 (73%, 66.9-78.1) |
| CR rate,∗ n (%, 95% CI†) | 136 (53%, 46.6-59.2) |
| Median time to first response (range),∗ mo | 0.95 (0.7-8.9) |
| Median time to first CR (range),∗ mo | 0.95 (0.8-12.5) |
| Median DOR (95% CI‡),∗mo | 23.1 (8.6-NR) |
| Median follow-up (95% CI§), mo | 23.0 (22.8-23.1) |
| Probability of continued response at 24 mo (95% CI‡), % | 49.5 (41.4-57.0) |
| Median DOR∗in patients who achieved CR (95% CI‡), mo | 26.1 (23.1-NR) |
| Median follow-up (95% CI§), mo | 23.1 (23.0-23.2) |
| Probability of continued response at 24 mo (95% CI‡), % | 58.5 (49.2-66.7) |
| Median PFS (95% CI‡),∗mo | 6.8 (3.3-12.7) |
| Median follow-up (95% CI§), mo | 23.9 (23.7-24.0) |
| Probability of PFS at 24 mo (95% CI‡), % | 40.6 (34.0-47.2) |
| Median OS (95% CI‡), mo | 27.3 (16.2-45.6) |
| Median follow-up (95% CI§), mo | 29.3 (26.2-30.4) |
| Probability of OS at 24 mo (95% CI‡), % | 50.5 (44.1-56.5) |
| . | Efficacy-evaluable set (N = 257) . |
|---|---|
| ORR,∗ n (%, 95% CI†) | 187 (73%, 66.9-78.1) |
| CR rate,∗ n (%, 95% CI†) | 136 (53%, 46.6-59.2) |
| Median time to first response (range),∗ mo | 0.95 (0.7-8.9) |
| Median time to first CR (range),∗ mo | 0.95 (0.8-12.5) |
| Median DOR (95% CI‡),∗mo | 23.1 (8.6-NR) |
| Median follow-up (95% CI§), mo | 23.0 (22.8-23.1) |
| Probability of continued response at 24 mo (95% CI‡), % | 49.5 (41.4-57.0) |
| Median DOR∗in patients who achieved CR (95% CI‡), mo | 26.1 (23.1-NR) |
| Median follow-up (95% CI§), mo | 23.1 (23.0-23.2) |
| Probability of continued response at 24 mo (95% CI‡), % | 58.5 (49.2-66.7) |
| Median PFS (95% CI‡),∗mo | 6.8 (3.3-12.7) |
| Median follow-up (95% CI§), mo | 23.9 (23.7-24.0) |
| Probability of PFS at 24 mo (95% CI‡), % | 40.6 (34.0-47.2) |
| Median OS (95% CI‡), mo | 27.3 (16.2-45.6) |
| Median follow-up (95% CI§), mo | 29.3 (26.2-30.4) |
| Probability of OS at 24 mo (95% CI‡), % | 50.5 (44.1-56.5) |
By IRC assessment per Lugano 2014 criteria.
Two-sided 95% exact Clopper-Pearson CIs.
Kaplan-Meier method was used to obtain the 2-sided 95% CIs.
Reverse Kaplan-Meier method was used to obtain the median follow-up and its 95% CIs.
Efficacy results in the efficacy-evaluable set. Kaplan-Meier curves demonstrating (A) DOR, (B) PFS, and (C) OS are shown. Responses were evaluated by IRC assessment per Lugano 2014 criteria. Kaplan-Meier method was used to calculate median (95% CI) of DOR, PFS, and OS; reverse Kaplan-Meier method was used to calculate median (95% CI) of follow-up. DOR and PFS only included data from TRANSCEND NHL 001. OS included survival data from patients who completed TRANSCEND NHL 001 and enrolled in the subsequent long-term follow-up study.
Efficacy results in the efficacy-evaluable set. Kaplan-Meier curves demonstrating (A) DOR, (B) PFS, and (C) OS are shown. Responses were evaluated by IRC assessment per Lugano 2014 criteria. Kaplan-Meier method was used to calculate median (95% CI) of DOR, PFS, and OS; reverse Kaplan-Meier method was used to calculate median (95% CI) of follow-up. DOR and PFS only included data from TRANSCEND NHL 001. OS included survival data from patients who completed TRANSCEND NHL 001 and enrolled in the subsequent long-term follow-up study.
By IRC assessment, 135 patients (53%) had PFS events while on the TRANSCEND study. The median PFS was 6.8 months (95% CI, 3.3-12.7) with a median follow-up of 23.9 months (95% CI, 23.7-24.0; Table 2; Figure 2B). The probability of 2-year PFS was 40.6% (95% CI, 34.0-47.2). The median PFS among patients who achieved CR was 27.3 months (95% CI, 24.0-NR).
OS analyses included survival follow-up data from patients in the efficacy-evaluable set while on TRANSCEND and additional data from the 81 patients who completed TRANSCEND and enrolled in the separate long-term follow-up study; 134 patients died while on TRANSCEND or during the long-term follow-up study. The median OS was 27.3 months (95% CI, 16.2-45.6) with a median follow-up for survival of 29.3 months (95% CI, 26.2-30.4; Table 2; Figure 2C). The probability of survival at 2 years was 50.5% (95% CI, 44.1-56.5). The median OS among patients who had a best response of CR was 48.5 months (95% CI, 45.2-NR; Figure 2C). Three late deaths occurred after 45 months: 2 patients remained in response upon death from unknown causes at 45 and 48 months, respectively, and 1 patient, whose disease had progressed at 3 months after liso-cel infusion in TRANSCEND according to the treating investigator, received multiple subsequent anticancer therapies and ultimately died because of septic shock in the setting of disease progression at 46 months.
Efficacy outcomes of all patients who had leukapheresis (intention-to-treat population; N = 345) are shown in supplemental Table 1, available on the Blood website.
Safety
The safety profile of liso-cel observed at 2 years included data from 1 additional patient treated with liso-cel since the prior analysis and was consistent with previous reports.10 In the treatment-emergent period (within 90 days of liso-cel infusion), 268 patients (99%) in the liso-cel–treated set (N = 270) experienced any-grade AEs; no new grade ≥3 AEs or serious AEs occurred since the previous analysis. Among patients in the liso-cel–treated set with safety data in the posttreatment-emergent period (N = 249), including data from patients who received retreatment with liso-cel (n = 17) and data from patients who completed TRANSCEND and enrolled in the long-term follow-up study (n = 81), 105 (42%) patients had AEs, with no individual AE reported in ≥10% of patients (Table 3). Similarly, incidences of grade ≥3 AEs and serious AEs were also substantially lower in the posttreatment-emergent period. In both the treatment-emergent and posttreatment-emergent periods, respectively, the most common any-grade AEs were neutropenia (63%; 8%), anemia (48%; 8%), and fatigue (44%; 7%), and the most common grade ≥3 AEs were cytopenias (neutropenia [60%; 7%], anemia [37%; 6%], and thrombocytopenia [27%; 4%]). In the posttreatment-emergent period, 5 patients had CRS that occurred after liso-cel retreatment (n = 17): 4 had low-grade events and 1 had a grade 3 event.
Summary of AEs during and after the TE period
| . | During TE period∗ (N = 270) . | Post-TE period† (N = 249) . | ||
|---|---|---|---|---|
| Patients with any-grade AEs, n (%) | 268 (99) | 105 (42) | ||
| Grade ≥3 | 213 (79) | 57 (23) | ||
| Grade 5 | 7 (3) | 5 (2) | ||
| Serious | 122 (45) | 42 (17) | ||
| Patients with most common AEs,‡n (%) | Any grade | Grade ≥3 | Any grade | Grade ≥3 |
| Neutropenia | 169 (63) | 161 (60) | 21 (8) | 17 (7) |
| Anemia | 129 (48) | 101 (37) | 19 (8) | 16 (6) |
| Fatigue | 119 (44) | 4 (1) | 18 (7) | 1 (<1) |
| CRS | 113 (42) | 6 (2) | 5 (2) | 1 (<1) |
| Nausea | 90 (33) | 4 (1) | 15 (6) | 0 |
| Thrombocytopenia | 85 (31) | 73 (27) | 16 (6) | 10 (4) |
| Headache | 80 (30) | 3 (1) | 8 (3) | 1 (<1) |
| Decreased appetite | 77 (29) | 7 (3) | 10 (4) | 1 (<1) |
| Diarrhea | 71 (26) | 1 (<1) | 13 (5) | 2 (<1) |
| Constipation | 63 (23) | 0 | 8 (3) | 0 |
| Dizziness | 60 (22) | 1 (<1) | 5 (2) | 0 |
| Hypotension | 60 (22) | 8 (3) | 5 (2) | 0 |
| Cough | 57 (21) | 0 | 6 (2) | 0 |
| Vomiting | 56 (21) | 1 (<1) | 7 (3) | 0 |
| Hypokalemia | 52 (19) | 6 (2) | 5 (2) | 0 |
| Hypomagnesemia | 50 (19) | 1 (<1) | 5 (2) | 0 |
| Abdominal pain | 45 (17) | 5 (2) | 6 (2) | 1 (<1) |
| Pyrexia | 45 (17) | 0 | 11 (4) | 0 |
| Leukopenia | 44 (16) | 39 (14) | 4 (2) | 2 (<1) |
| Peripheral edema | 42 (16) | 1 (<1) | 6 (2) | 1 (<1) |
| Sinus tachycardia | 42 (16) | 0 | 3 (1) | 0 |
| Tremor | 41 (15) | 0 | 0 | 0 |
| Confusional state | 39 (14) | 2 (<1) | 4 (2) | 0 |
| Hypertension | 37 (14) | 12 (4) | 0 | 0 |
| Hypogammaglobulinemia§ | 37 (14) | 0 | 13 (5) | 0 |
| Dyspnea | 36 (13) | 2 (<1) | 4 (2) | 1 (<1) |
| Insomnia | 36 (13) | 1 (<1) | 9 (4) | 0 |
| Back pain | 33 (12) | 3 (1) | 3 (1) | 1 (<1) |
| Chills | 31 (11) | 0 | 4 (2) | 0 |
| Anxiety | 27 (10) | 0 | 5 (2) | 0 |
| Hypophosphatemia | 27 (10) | 16 (6) | 3 (1) | 2 (<1) |
| Febrile neutropenia | 25 (9) | 24 (9) | 10 (4) | 9 (4) |
| Myelodysplastic syndrome | 1 (<1) | 1 (<1) | 7 (3) | 7 (3) |
| Basal cell carcinoma | 1 (<1) | 0 | 5 (2) | 2 (<1) |
| . | During TE period∗ (N = 270) . | Post-TE period† (N = 249) . | ||
|---|---|---|---|---|
| Patients with any-grade AEs, n (%) | 268 (99) | 105 (42) | ||
| Grade ≥3 | 213 (79) | 57 (23) | ||
| Grade 5 | 7 (3) | 5 (2) | ||
| Serious | 122 (45) | 42 (17) | ||
| Patients with most common AEs,‡n (%) | Any grade | Grade ≥3 | Any grade | Grade ≥3 |
| Neutropenia | 169 (63) | 161 (60) | 21 (8) | 17 (7) |
| Anemia | 129 (48) | 101 (37) | 19 (8) | 16 (6) |
| Fatigue | 119 (44) | 4 (1) | 18 (7) | 1 (<1) |
| CRS | 113 (42) | 6 (2) | 5 (2) | 1 (<1) |
| Nausea | 90 (33) | 4 (1) | 15 (6) | 0 |
| Thrombocytopenia | 85 (31) | 73 (27) | 16 (6) | 10 (4) |
| Headache | 80 (30) | 3 (1) | 8 (3) | 1 (<1) |
| Decreased appetite | 77 (29) | 7 (3) | 10 (4) | 1 (<1) |
| Diarrhea | 71 (26) | 1 (<1) | 13 (5) | 2 (<1) |
| Constipation | 63 (23) | 0 | 8 (3) | 0 |
| Dizziness | 60 (22) | 1 (<1) | 5 (2) | 0 |
| Hypotension | 60 (22) | 8 (3) | 5 (2) | 0 |
| Cough | 57 (21) | 0 | 6 (2) | 0 |
| Vomiting | 56 (21) | 1 (<1) | 7 (3) | 0 |
| Hypokalemia | 52 (19) | 6 (2) | 5 (2) | 0 |
| Hypomagnesemia | 50 (19) | 1 (<1) | 5 (2) | 0 |
| Abdominal pain | 45 (17) | 5 (2) | 6 (2) | 1 (<1) |
| Pyrexia | 45 (17) | 0 | 11 (4) | 0 |
| Leukopenia | 44 (16) | 39 (14) | 4 (2) | 2 (<1) |
| Peripheral edema | 42 (16) | 1 (<1) | 6 (2) | 1 (<1) |
| Sinus tachycardia | 42 (16) | 0 | 3 (1) | 0 |
| Tremor | 41 (15) | 0 | 0 | 0 |
| Confusional state | 39 (14) | 2 (<1) | 4 (2) | 0 |
| Hypertension | 37 (14) | 12 (4) | 0 | 0 |
| Hypogammaglobulinemia§ | 37 (14) | 0 | 13 (5) | 0 |
| Dyspnea | 36 (13) | 2 (<1) | 4 (2) | 1 (<1) |
| Insomnia | 36 (13) | 1 (<1) | 9 (4) | 0 |
| Back pain | 33 (12) | 3 (1) | 3 (1) | 1 (<1) |
| Chills | 31 (11) | 0 | 4 (2) | 0 |
| Anxiety | 27 (10) | 0 | 5 (2) | 0 |
| Hypophosphatemia | 27 (10) | 16 (6) | 3 (1) | 2 (<1) |
| Febrile neutropenia | 25 (9) | 24 (9) | 10 (4) | 9 (4) |
| Myelodysplastic syndrome | 1 (<1) | 1 (<1) | 7 (3) | 7 (3) |
| Basal cell carcinoma | 1 (<1) | 0 | 5 (2) | 2 (<1) |
Most common AEs are listed in descending order of incidence in the TE period.
TE, treatment-emergent.
The TE period started any time from initiation of liso-cel administration to and including 90 days after liso-cel infusion. Any AE occurring after the initiation of another anticancer therapy or liso-cel retreatment were not considered as a TEAE.
The posttreatment-emergent period started from 91 days after liso-cel infusion or from initiation of another anticancer therapy or liso-cel retreatment (if patients initiated another anticancer therapy or liso-cel retreatment before 91 days after liso-cel infusion).
Any-grade AEs occurring in ≥10% in the TE period or ≥2% in the post-TE period.
Includes events occurring after liso-cel infusion coded to the following Medical Dictionary for Regulatory Activities preferred terms: blood IgA decreased, blood IgD decreased, blood IgE decreased, blood IgG decreased, blood IgM decreased, hypogammaglobulinemia, Igs decreased, selective IgA immunodeficiency, selective IgG subclass deficiency, and selective IgM immunodeficiency.
In the treatment-emergent period, 127 patients (47%) in the liso-cel–treated set experienced CRS or NEs; no grade 5 CRS or NEs were reported (Table 4). Any-grade CRS was reported in 113 (42%) patients, with grade 3 or 4 events in only 6 patients (2%). The median time to onset of CRS was 5 days, as was the median time to resolution. A total of 53 patients (20%) received tocilizumab and/or corticosteroids for the treatment of CRS, including 27 (10%) who received tocilizumab only, 5 (2%) who received corticosteroids only, and 21 (8%) who received both tocilizumab and corticosteroids. Seven patients (3%) also received vasopressors for the treatment of CRS, and 1 (<1%) received an additional immunosuppressive agent. Eighty (30%) patients had NEs of any grade, and 27 (10%) patients had grade 3 or 4 NEs. The median times to onset and resolution of NE were 9 days and 11 days, respectively. A total of 45 (17%) patients received tocilizumab and/or corticosteroids for the treatment of NEs, including 36 (13%) who received corticosteroids only, 1 (<1%) who received tocilizumab only, and 8 (3%) who received both tocilizumab and corticosteroids. One patient (<1%) also received vasopressors for the treatment of NEs, and 2 (<1%) received an additional immunosuppressive agent.
AEs of special interest during the TE period
| . | Liso-cel–treated set (N = 270) . |
|---|---|
| Patients with CRS or NE, n (%) | 127 (47) |
| Patients with CRS,∗n (%) | |
| Any grade | 113 (42) |
| Grade 1 | 65 (24) |
| Grade 2 | 42 (16) |
| Grade 3 | 4 (1) |
| Grade 4 | 2 (1) |
| Grade 5 | 0 |
| Median time to CRS onset (range), d | 5 (1-14) |
| Median time to resolution of CRS (range), d | 5 (1-17) |
| Treatment with tocilizumab and/or corticosteroids for CRS, n (%) | |
| Tocilizumab only | 27 (10) |
| Corticosteroids only | 5 (2) |
| Both tocilizumab and corticosteroids | 21 (8) |
| Patients with NE,†n (%) | |
| Any grade | 80 (30) |
| Grade 1 | 26 (10) |
| Grade 2 | 27 (10) |
| Grade 3 | 23 (9) |
| Grade 4 | 4 (1) |
| Grade 5 | 0 |
| Median time to NE onset (range), d | 9 (1-66) |
| Median time to resolution of NE (range), d | 11 (1-86) |
| Treatment with tocilizumab and/or corticosteroids for NEs, n (%) | |
| Tocilizumab only | 1 (<1) |
| Corticosteroids only | 36 (13) |
| Both tocilizumab and corticosteroids | 8 (3) |
| Patients with prolonged cytopenia‡on d 29, n (%) | 101 (37) |
| Patients with grade 3-4 decreased Hb, n (%) | 17 (6) |
| Median time of duration to recovery§ to grade ≤2 in 10 patients (range), d | 21 (3-72) |
| Patients with grade 3-4 decreased platelets, n (%) | 81 (30) |
| Median time of duration to recovery§ to grade ≤2 in 48 patients (range), d | 29.5 (1-328) |
| Patients with grade 3-4 decreased neutrophils, n (%) | 53 (20) |
| Median time of duration to recovery§ to grade ≤2 in 42 patients (range), d | 25.5 (2-336) |
| Infections,‖n (%) | |
| Any grade | 111 (41) |
| Grade ≥3 | 33 (12) |
| . | Liso-cel–treated set (N = 270) . |
|---|---|
| Patients with CRS or NE, n (%) | 127 (47) |
| Patients with CRS,∗n (%) | |
| Any grade | 113 (42) |
| Grade 1 | 65 (24) |
| Grade 2 | 42 (16) |
| Grade 3 | 4 (1) |
| Grade 4 | 2 (1) |
| Grade 5 | 0 |
| Median time to CRS onset (range), d | 5 (1-14) |
| Median time to resolution of CRS (range), d | 5 (1-17) |
| Treatment with tocilizumab and/or corticosteroids for CRS, n (%) | |
| Tocilizumab only | 27 (10) |
| Corticosteroids only | 5 (2) |
| Both tocilizumab and corticosteroids | 21 (8) |
| Patients with NE,†n (%) | |
| Any grade | 80 (30) |
| Grade 1 | 26 (10) |
| Grade 2 | 27 (10) |
| Grade 3 | 23 (9) |
| Grade 4 | 4 (1) |
| Grade 5 | 0 |
| Median time to NE onset (range), d | 9 (1-66) |
| Median time to resolution of NE (range), d | 11 (1-86) |
| Treatment with tocilizumab and/or corticosteroids for NEs, n (%) | |
| Tocilizumab only | 1 (<1) |
| Corticosteroids only | 36 (13) |
| Both tocilizumab and corticosteroids | 8 (3) |
| Patients with prolonged cytopenia‡on d 29, n (%) | 101 (37) |
| Patients with grade 3-4 decreased Hb, n (%) | 17 (6) |
| Median time of duration to recovery§ to grade ≤2 in 10 patients (range), d | 21 (3-72) |
| Patients with grade 3-4 decreased platelets, n (%) | 81 (30) |
| Median time of duration to recovery§ to grade ≤2 in 48 patients (range), d | 29.5 (1-328) |
| Patients with grade 3-4 decreased neutrophils, n (%) | 53 (20) |
| Median time of duration to recovery§ to grade ≤2 in 42 patients (range), d | 25.5 (2-336) |
| Infections,‖n (%) | |
| Any grade | 111 (41) |
| Grade ≥3 | 33 (12) |
The TE period started any time from initiation of liso-cel administration through and including 90 days after liso-cel infusion. Any AE occurring after the initiation of another anticancer therapy or liso-cel retreatment were not considered as a TEAE.
CRS was graded per Lee 2014 criteria.21
NEs were defined as investigator-identified neurological AEs related to liso-cel and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Prolonged cytopenia is defined as any grade ≥3 laboratory result of decreased Hb, decreased neutrophil count, or decreased platelet count on the day 29 visit. The protocol-defined window for the day 29 visit was 29 ± 2 days after liso-cel infusion. If multiple test results were available in the window, the maximum grade was selected.
The duration of grade 3 to 4 decreased hematology values was calculated as the date of the first grade ≤2 laboratory result after the day 29 visit‒day 29 visit date. Grade ≤2 laboratory results taken within 7 days after red blood cell or platelet transfusion were excluded. Recovery data are presented for patients who had hematology laboratory results after day 29.
Includes all events in the infections and infestations System Organ Class.
In the treatment-emergent period, prolonged cytopenia, defined as grade ≥3 laboratory results of decreased Hb, platelet count, or neutrophil count at the day 29 visit, was reported for 101 patients (37%) in the liso-cel–treated set, including 17 (6%) with decreased Hb, 81 (30%) with decreased platelets, and 53 (20%) with decreased neutrophils (Table 4). Among patients with prolonged cytopenia who had hematology laboratory results available after day 29, recovery to grade 2 or better occurred in 10 of 11 (91%) patients with anemia, 48 of 59 (81%) patients with thrombocytopenia, and 42 of 44 (95%) patients with neutropenia, with median times to recovery of 21 (range, 3-72), 29.5 (range, 1-328), and 25.5 (range, 2-336) days, respectively.
AEs of any grade in the infections and infestations System Organ Class occurred in 111 patients (41%) in the treatment-emergent period (grade ≥3, n = 33 [12%]) and in 24 patients (10%; grade ≥3, n = 12 [5%]) in the posttreatment-emergent period. The most common infections were pneumonia (7%), upper respiratory tract infection (4%), and Candida infection (4%) in the treatment-emergent period (N = 270) and pneumonia (2%), oral candidiasis (2%), and Candida infection (<1%) in the posttreatment-emergent period. Three patients (1%) had treatment-emergent AEs of cytomegalovirus (CMV) infection reactivation (grade ≥3, n = 1), 2 (1%) had events of CMV viremia (none grade ≥3), and 1 (<1%) had an event of CMV infection (none grade ≥3). No CMV-related AEs were reported in the posttreatment-emergent period. No COVID-19 infections were reported in the treatment-emergent or posttreatment-emergent periods.
Of the 259 patients in the liso-cel–treated set who had serum immunoglobulin G (IgG) laboratory data available, IgG <500 mg/dL was observed in 127 of 259 patients (49%) at baseline and did not change substantially over time after liso-cel administration; on day 29, 6 months, 1 year, and 2 years after liso-cel administration, IgG <500 mg/dL was observed in 137 of 238 (58%), 104 of 157 (66%), 77 of 128 (60%), and 53 of 93 (57%) patients, respectively. During the treatment-emergent and posttreatment-emergent periods, hypogammaglobulinemia was reported as an AE in 37 (14%) and 13 (5%) patients, respectively.
All new malignancies occurring on the studies were required to be reported. Second primary malignancies (SPMs) were reported for 5 patients (2%) during the treatment-emergent period and 17 patients (7%) during the posttreatment-emergent period through 24 months. The most common SPM was nonmelanoma skin cancer in 9 patients. All SPMs through 24 months are listed in supplemental Table 2, and cumulative incidence of hematologic SPMs is shown in supplemental Figure 1. Hematologic SPMs across both treatment periods included myelodysplastic syndrome in 8 patients and acute myeloid leukemia in 2 patients. One case of peripheral T-cell lymphoma occurred on day 30. Liso-cel vector transgene testing of the peripheral T-cell lymphoma showed negative results, ruling out its relationship with liso-cel. Retrospective disease confirmation by the central laboratory identified a T-cell component in the tumor biopsy sample taken before liso-cel infusion.
A total of 133 patients (49%) died after receiving liso-cel treatment; 110 died from disease progression, mostly (86 patients) during the posttreatment-emergent period (supplemental Table 3). Eleven (4%) deaths due to AEs (progressive multifocal leukoencephalopathy and septic shock [n = 2 each]; pulmonary hemorrhage, multiple organ dysfunction, diffuse alveolar damage, leukoencephalopathy, cardiomyopathy, myelodysplastic syndrome, and acute myeloid leukemia [n = 1 each]) occurred anytime on study, including 8 patients (3%) during the treatment-emergent period and 3 patients (1%) during the posttreatment-emergent period. Diffuse alveolar damage, pulmonary hemorrhage, multiple organ dysfunction, cardiomyopathy, and, in the posttreatment-emergent period, 1 case of progressive multifocal leukoencephalopathy were considered related to both liso-cel and lymphodepleting chemotherapy by the investigator. Acute myeloid leukemia, which was attributed as related to liso-cel but not lymphodepleting chemotherapy by the treating investigator, occurred ∼18 months after infusion in a patient who was heavily pretreated with agents known to be associated with secondary malignancies including acute myeloid leukemia, including 4 immunochemotherapy regimens and high-dose chemotherapy followed by autologous stem cell transplantation.26-28 One additional patient who received liso-cel had a posttreatment-emergent grade 5 event of death that occurred after retreatment with liso-cel, in which the primary cause of death was reported as “unknown” rather than “due to AE.” No patient died of COVID-19.
Cellular kinetics
CAR T-cell persistence rate was 37% (26 of 70 evaluable patients) at 24 months and 43% (3 of 7 evaluable patients) at 42 months (supplemental Table 4). No apparent differences in transgene persistence were observed up to 2 years in responders, regardless of relapse status.
Discussion
To our knowledge, TRANSCEND is the largest clinical study reported to date of CD19-directed CAR T-cell treatment for patients with R/R LBCL. At 2-years of follow-up, liso-cel demonstrated durable clinical activity with 24-month PFS and OS rates of 40.6% and 50.5%, respectively, in a broad population of patients. The ORR was 73% with 53% of patients achieving a best response of CR. The median DOR, PFS, and OS was 23.1 months, 6.8 months, and 27.3 months, respectively, in the liso-cel–treated efficacy set. These long-term follow-up results confirm durable remissions in a broad range of patients with R/R LBCL who had ≥2 prior treatments, high-risk disease features, and diverse histologic subtypes and for whom the estimated median OS with conventional salvage chemotherapy was substantially shorter at 6 to 10 months.3,29
No new safety signals were observed during this 2-year follow-up, and few AEs occurred after the 90-day treatment-emergent period. As previously reported for the primary analysis and confirmed in this 2-year follow-up, liso-cel infusion was associated with low incidences of severe CRS or NEs, late onset of CRS and NE, and low use of tocilizumab and corticosteroids.10 The proportion of patients who experienced prolonged cytopenias in this study was similar to previous reports of patients who received CAR T-cell therapy.30,31 Among patients with prolonged cytopenia who had laboratory results after day 29 and recovered to grade ≤2 laboratory values, most recovered in ∼1 month. The incidence of grade ≥3 infections was similar or lower compared with incidences in other long-term CAR T-cell therapy studies,30,32 further highlighting the manageable safety profile of liso-cel. Repeated assessment for any new cancers is an important component of long-term care after CAR T-cell therapy.33 SPMs were infrequent in this study and were within the rates expected for a heavily pretreated patient population.
The long-term efficacy data for TRANSCEND compare favorably with those of other CD19-directed CAR T-cell therapies.30,32 In long-term results from the ZUMA-1 study evaluating the CD19-directed CAR T-cell therapy axicabtagene ciloleucel as third-line or later (3L+) therapy for 101 patients with R/R LBCL followed up for a median of 27.1 months, the ORR was 83% (CR rate, 58%) and the median DOR was 11.1 months by investigator assessment but NR by IRC assessment.32 The median PFS by investigator assessment was 5.9 months, and the median OS was NR.32 The estimated 24-month PFS rates were 72%, 75%, and 22% among patients with CR, PR, and stable disease at 3 months, respectively. Grade ≥3 CRS and NEs occurred in 11% and 32% of patients, respectively. In an additional analysis of the ZUMA-1 study (median follow-up, 51.1 months), the median OS was 25.8 months and the 4-year OS rate was 44%, with no new safety signals reported.34 At a median follow-up of 40.3 months for 115 patients with R/R LBCL treated with tisagenlecleucel as a 3L+ therapy in the long-term analysis of the JULIET study, the ORR was 53% (CR rate, 39%) and the median DOR was NR.30 The estimated proportion of patients with a response at 36 months after response onset was 60.4%. The median PFS and OS was 2.9 months and 11.1 months, respectively.30 The estimated 24-month PFS rates were 75% and 83% among patients with CR at 3 and 6 months, respectively. Grade ≥3 CRS and NEs occurred in 23% and 11% of patients, respectively. Altogether, long-term data from the TRANSCEND, ZUMA-1, and JULIET studies with ∼2 to 4 years of follow-up demonstrate that CAR T-cell therapies offer the opportunity for cure in patients with R/R LBCL after ≥2 prior lines of therapy. Although cross-trial comparisons are difficult due to the use of different toxicity grading scales, among these 3 pivotal trials, liso-cel reported the lowest rates of any-grade and grade ≥3 CRS (Lee criteria),21 and demonstrated any-grade and grade ≥3 NE incidences lower than axicabtagene ciloleucel and comparable with tisagenlecleucel, indicating a manageable long-term safety profile.8,10,35 Of note, the Penn grading system for CRS36 used in JULIET results in higher rates of severe CRS relative to the Lee criteria used in TRANSCEND and ZUMA-1. Matching-adjusted indirect comparisons of liso-cel vs axicabtagene ciloleucel and tisagenlecleucel as a 3L+ treatment of R/R LBCL indicate that liso-cel has comparable efficacy and a more manageable safety profile than axicabtagene ciloleucel37 and favorable efficacy with a comparable or better safety profile than tisagenlecleucel,38 further supporting the long-term results of TRANSCEND.
This study has some limitations. As with the other pivotal trials of CAR T cells in 3L+ LBCL, this study design did not include a comparator arm; therefore, no direct comparisons with other treatments can be made. However, an analysis comparing results from TRANSCEND to a real-world synthetic, matched control arm of patients who received conventional therapies demonstrated a statistically significant and clinically meaningful benefit with liso-cel over conventional therapies in patients with 3L+ R/R LBCL.39 The TRANSCEND study was designed to follow patients for 2 years after liso-cel treatment, after which patients were asked to enroll in a separate long-term follow-up study assessing safety and OS for up to 15 years. The requirement of a separate consent form for patients to enter the long-term follow-up study limited the number of patients enrolling in subsequent long-term follow-up. Of patients treated with liso-cel who were alive and completed follow-up in TRANSCEND (n = 120) or withdrew early from the study (n = 10), 81 patients enrolled in the long-term follow-up study. In addition, as IRC assessments were performed only in the 2-year TRANSCEND study period, DOR and PFS were censored at the completion of TRANSCEND, and therefore, DOR and PFS per IRC were likely underestimated as evidenced by the high number of censoring events at 24 months on both Kaplan-Meier curves.
In conclusion, results from this 2-year follow-up of the TRANSCEND study demonstrated durable efficacy and a manageable safety profile of liso-cel for patients with R/R LBCL, including multiple histologic subtypes and high-risk features. Responses to liso-cel were durable, with a long median DOR and high DOR, PFS, and OS rates at 24 months. Few AEs were reported after the initial 90-day treatment-emergent period, and no new safety signals were observed during the 2-year follow-up. Additional studies are evaluating liso-cel in other B-cell leukemias and lymphomas.
Acknowledgments
Writing and editorial assistance was provided by Bu Reinen and Allison Green of The Lockwood Group (Stamford, CT), funded by Bristol Myers Squibb.
The TRANSCEND NHL 001 study was funded by Juno Therapeutics, a Bristol Myers Squibb Company, and the long-term follow-up study was funded by Celgene, a Bristol Myers Squibb Company.
Authorship
Contribution: J.S.A. contributed to data acquisition and data interpretation; M.L.P., L.I.G., M.L., M.W., J.A., E.P., C.A., A.S., S.R.S., N.G., and T.S. contributed to data acquisition; D.G.M. contributed to study concept or design and data acquisition; C.D. contributed to study concept or design, data analysis, and data interpretation; Y.K., K.O., and A.K. contributed to data analysis and data interpretation; J.S.A. and A.K. confirmed the report with contributions and critical revisions from all authors; J.S.A., C.D., and Y.K. verified the underlying data; and all authors had full access to all data, contributed to and approved the final version to be published, and agreed to be accountable for all aspects of the work.
Conflict-of-interest disclosure: J.S.A. declares consultancy from AbbVie, Allogene Therapeutics, AstraZeneca, BeiGene, Bluebird Bio, Bristol Myers Squibb, C4 Therapeutics, Celgene, a Bristol Myers Squibb Company, Eli Lilly, EMD Serono Inc, Genentech, a member of the Roche Group, Genmab, Incyte Corporation, Karyopharm Therapeutics, Kite Pharma, Kymera, MorphoSys, Novartis, and Regeneron; and research grants from Bristol Myers Squibb and Seagen Inc. M.L.P. declares consultancy from Novartis, Kite Pharma, Pharmacyclics, BeiGene; stock from Seres and Notch; research funding from Seres; patents and royalties from Seres, Juno Therapeutics, a Bristol Myers Squibb Company, and Wolters Kluwer; and honoraria from Seres, Notch, Magenta, WindMIL, Rheos, Nektar, Priothera, Ceramedix, Lygenesis, and Pluto. L.I.G. declares patents (no royalties) from Zylem Biosciences; and honoraria and research funding from Bristol Myers Squibb. M.L. declares consultancy from Kite (a Gilead company), Celgene, a Bristol Myers Squibb Company, Verastem, Janssen, Myeloid Therapeutics, AstraZeneca, Acrotech, ADC Therapeutics, Legend, Spectrum, BeiGene, Daiichi Sankyo, MorphoSys, TG Therapeutics, Novartis, Kyowa Kirin, Karyopharm Therapeutics, and AbbVie. M.W. declares consultancy from AstraZeneca, Bayer Healthcare, BeiGene, CSTone, DTRM Biopharma (Cayman) Limited, Epizyme, Genentech, Innocare, Janssen, Juno Therapeutics, a Bristol Myers Squibb Company, Kite Pharma, Loxo Oncology, Miltenyi Biomedicine GmbH, Newbridge Pharmaceuticals, Oncternal, Pharmacyclics, and VelosBio; honoraria from AstraZeneca, Acerta Pharma, Anticancer Association, BeiGene, Chinese American Hematologist and Oncologist Network, Chinese Medical Association, Clinical Care Options, DAVA Oncology, Epizyme, Hebei Cancer Prevention Federation, Imbruvica, Imedex, Janssen, Kite Pharma, Miltenyi Biomedicine GmbH, Moffitt Cancer Center, Mumbai Hematology Group, Newbridge Pharmaceuticals, Omi Pharma, Physicians Education Resources, Scripps, and The First Afflicted Hospital of Zhejiang University; and research funding from AstraZeneca, Acerta Pharma, BeiGene, BioInvent, Juno/Celgene, a Bristol Myers Squibb Company, Innocare, Janssen, Kite Pharma, Lilly, Loxo Oncology, Molecular Templates, Oncternal Therapeutics, Pharmacyclics, and VelosBio. J.A. declares honoraria from Juno Therapeutics, a Bristol Myers Squibb Company. D.G.M. declares honoraria from A2 Biotherapeutics, Navan Technologies, Kite Pharma, Amgen, Bristol Myers Squibb, Juno/Celgene, a Bristol Myers Squibb Company, MorphoSys, Novartis, Genentech, Legend Biotech, Janssen, and Umoja; stock from A2 Biotherapeutics, Navan Technologies; institutional research funding from Kite Pharma and Juno/Celgene, a Bristol Myers Squibb Company; and rights to royalties from Fred Hutchinson Cancer Research Center for patents licensed to Bristol Myers Squibb and Juno/Celgene, a Bristol Myers Squibb Company. C.A. declares research funding from Merck, Bristol Myers Squibb, CRISPR Therapeutics, Genmab, and Novartis; is a current equity holder and former employee of Roche; consultancy from Atara; and receives honoraria from Epizyme, Incyte, TG Therapeutics, Kite Pharma, Karyopharm Therapeutics, and Atara. A.S. declares research funding from Kite (a Gilead company), Juno/Celgene, a Bristol Myers Squibb Company. N.G. declares consultancy fees from Janssen, Kite Pharma, Genmab, Incyte, Bristol Myers Squibb, BeiGene, TG Therapeutics, ADC Therapeutics, Adaptive Biotech, AbbVie, and Pharmacyclics; speakers’ bureau fees from Janssen, Kite Pharma, Epizyme, Bristol Myers Squibb, AbbVie, and Pharmacyclics; and research funding from Bristol Myers Squibb, TG Therapeutics, Genentech, and Pharmacyclics. A.K., K.O., and C.D. are current employees and equity holders at Bristol Myers Squibb. Y.K. was an employee of Bristol Myers Squibb at the time the study was conducted. T.S. declares membership on an entity’s advisory committees and speakers’ bureau as well as research funding from AstraZeneca, BeiGene, Bristol Myers Squibb, Juno Therapeutics, a Bristol Myers Squibb Company, Kite Pharma, Pharmacyclics (an AbbVie Company), Celgene, a Bristol Myers Squibb Company; and research funding from Oncternal Therapeutics, TG Therapeutics, and Ascentage Pharma. The remaining authors declare no competing financial interests.
Correspondence: Jeremy S. Abramson, Lymphoma Program, Massachusetts General Hospital Cancer Center, Harvard Medical School, 55 Fruit St, Boston, MA 02114; email: jabramson@mgh.harvard.edu.
References
Author notes
∗J.S.A. and M.L.P. contributed equally to this publication.
The current affiliation for author Y.K. is Day One Biopharmaceuticals (Brisbane, CA, USA).
Presented in abstract form as a poster at the 63rd annual meeting and exposition of the American Society of Hematology, 11-14 December 2021.
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal