Tumor-agnostic ctDNA sequencing in CNSL is feasible and allows for detection of PRD.
We propose the molecular prognostic index for CNSL, a model integrating clinical and molecular features for improved risk profiling in CNSL.
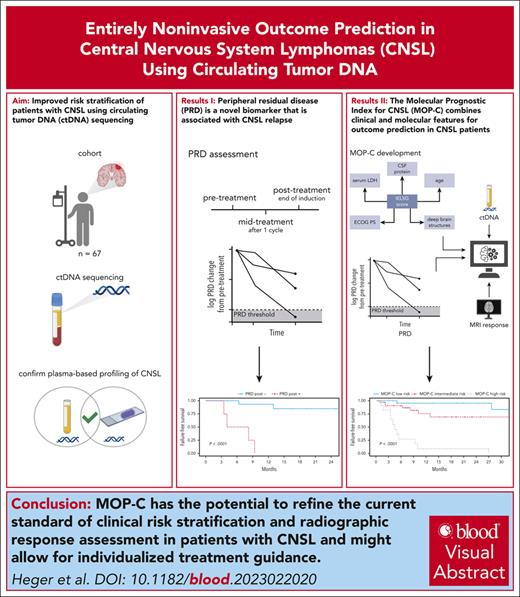
Visual Abstract
State-of-the-art response assessment of central nervous system lymphoma (CNSL) by magnetic resonance imaging is challenging and an insufficient predictor of treatment outcomes. Accordingly, the development of novel risk stratification strategies in CNSL is a high unmet medical need. We applied ultrasensitive circulating tumor DNA (ctDNA) sequencing to 146 plasma and cerebrospinal fluid (CSF) samples from 67 patients, aiming to develop an entirely noninvasive dynamic risk model considering clinical and molecular features of CNSL. Our ultrasensitive method allowed for the detection of CNSL-derived mutations in plasma ctDNA with high concordance to CSF and tumor tissue. Undetectable plasma ctDNA at baseline was associated with favorable outcomes. We tracked tumor-specific mutations in plasma-derived ctDNA over time and developed a novel CNSL biomarker based on this information: peripheral residual disease (PRD). Persistence of PRD after treatment was highly predictive of relapse. Integrating established baseline clinical risk factors with assessment of radiographic response and PRD during treatment resulted in the development and independent validation of a novel tool for risk stratification: molecular prognostic index for CNSL (MOP-C). MOP-C proved to be highly predictive of outcomes in patients with CNSL (failure-free survival hazard ratio per risk group of 6.60; 95% confidence interval, 3.12-13.97; P < .0001) and is publicly available at www.mop-c.com. Our results highlight the role of ctDNA sequencing in CNSL. MOP-C has the potential to improve the current standard of clinical risk stratification and radiographic response assessment in patients with CNSL, ultimately paving the way toward individualized treatment.
Introduction
Involvement of the central nervous system (CNS) by aggressive B-cell lymphomas is a rare event demanding special diagnostic and therapeutic approaches.1 Although primary CNS lymphoma (PCNSL) is restricted to the CNS at diagnosis,2,3 secondary CNSL (SCNSL) comprises concurrent involvement of systemic and CNS sites at diagnosis as well as either isolated or concurrent relapse of systemic lymphoma within the CNS after treatment.4,5 Standard of care consists of systemic treatment based on high-dose methotrexate (MTX) combined with additional chemoimmunotherapeutic drugs.1-3,6-9 Magnetic resonance imaging (MRI) is commonly used to assess treatment efficacy but is highly limited because of insufficient discrimination between reactive and resorptive tissue alterations and vital tumor, leading to a low predictive performance.10 Most patients initially respond to treatment as determined by standardized imaging criteria.11-13 Conversion from partial remission (PR) to complete remission (CR) over time seems to be crucial for durable responses but is inherently a prognostic marker that is available only very late. Additionally, elderly patients often fail to achieve CR.12,14 In nonresponders, progressive disease (PD) will frequently result in early death.14 In peripheral lymphomas, a biopsy is performed in case of suspected relapse or equivocal imaging findings; however, because of its intracranial localization, serial biopsies are often not feasible in CNSL. Accordingly, noninvasive risk profiling strategies for patients with CNSL are a high unmet medical need.
We, and others, have shown that PCNSL and, albeit less frequently, SCNSL are characterized by recurrent mutations in genes such as MYD88, PIM1, and CD79B,15-18 resembling aggressive B-cell lymphoma subtypes cluster 5 or MCD, as defined in recent reports.19-21 High-throughput sequencing of circulating tumor DNA (ctDNA) is a promising tool to overcome the need for invasive tissue biopsies and has recently been successfully applied to aggressive B-cell lymphoma.22-27 However, previous approaches in CNSL suffered from low discovery rates.28-30 Beyond genotyping, assessment of minimal residual disease by ctDNA sequencing has been proven to be a valuable tool for response evaluation in aggressive B-cell lymphoma.31-35 Recently, Mutter et al. presented the first study to demonstrate the potential utility of ctDNA for noninvasive discrimination between CNSL and other primary brain tumors.36
However, data on the application of tumor-agnostic, ultrasensitive ctDNA sequencing in CNSL remains scarce. Here, we present an approach in which minimal residual disease assessment is performed using solely plasma ctDNA without requiring tumor tissue. We validate the latter as a novel biomarker and name it “peripheral residual disease” (PRD). Finally, we incorporate baseline clinical features and ctDNA levels at baseline as well as radiographic response assessment and PRD during treatment into a novel prognostic model for CNSL called the molecular prognostic index for CNSL (MOP-C).
Materials and methods
Ethical considerations
Samples were collected from patients treated at the University Hospitals of Cologne and Essen, Germany. All patients provided written informed consent. Sample collection and storage were approved by the local ethics committee of the University Hospitals of Cologne and Essen (nos. 13-091 and 11-481-BO, respectively). Sample analysis and extraction of clinical data from patient charts and the local cancer registry were approved by the local ethics committee of the University Hospitals Cologne and Essen (nos. 20-1579 and 22-10652-BO, respectively).
Patients and sample collection
We included all consecutively managed patients with histologically proven diagnosis of PCNSL or SCNSL and eligibility for systemic treatment containing high-dose MTX based on organ function as defined within the trials of the International Extranodal Lymphoma Study Group (IELSG).11,12 SCNSL comprises concurrent involvement of systemic and CNS sites at diagnosis as well as either isolated or concurrent relapse of systemic lymphoma within the CNS after treatment.4,5 Patients were treated according to local guidelines that were harmonized between the reporting institutions (University Hospital Cologne and University Hospital Essen) between January 2018 and September 2022 (details can be found in supplemental Methods, available on the Blood website). Information on patient and treatment characteristics was collected during treatment. Blood samples were collected at predetermined time points: (1) before initiation of systemic treatment except for the use of corticosteroids after stereotactic biopsy (pretreatment); (2) after 1 cycle of treatment (midtreatment); and (3) at the end of induction treatment (posttreatment). In case of relapse, another sample before secondline treatment and further follow-up samples were collected. Cerebrospinal fluid (CSF) samples were collected before treatment. A detailed sample breakdown is provided in supplemental Figure 1. Tumor samples for DNA extraction and targeted gene panel sequencing to compare mutations identified in plasma-derived ctDNA with mutational profiles obtained from tumor tissue were available for a subset of 13 patients. One of these samples failed quality criteria for sequencing because of low amounts of DNA and was, thus, excluded from the analysis.
Sample processing and DNA extraction
Sample processing and DNA extraction were performed as previously reported.37 Details can be found in supplemental Methods.
DNA processing, quality control, and sequencing
For target enrichment, we used customized RNA baits. We targeted 1.516 megabases of the human genome (supplemental Table 1). Our targeted gene panel was designed heuristically by including recurrent locations of somatic mutations and copy number variations based on previous studies exploring the mutational landscape in PCNSL by targeted gene panel sequencing29 and whole exome sequencing18 as well as previous studies describing genetic clusters of diffuse large B-cell lymphoma (DLBCL) by whole exome sequencing.19-21 Additionally, somatically hypermutated intergenic regions were added to increase the average number of available mutations for assessment of residual disease in follow-up samples.35 Additional information on DNA processing, quality control, and sequencing can be found in the supplemental Methods.
Basic data processing, somatic single base substitution, small insertion and deletion calling, and ctDNA quantification
Methods used for basic data processing, somatic single base substitution, small insertion and deletion calling, and ctDNA quantification can be found in supplemental Methods.
PRD measurement
PRD was measured using customized R scripts as previously described.37 Details on PRD measurement can be found in the supplemental Methods. PRD was assessed in all midtreatment and posttreatment samples.
Development and validation of MOP-C
The IELSG risk score and achievement of CR after completion of induction treatment as assessed by MRI have been proven to possess predictive value in CNSL.38,39 We hypothesized that incorporation of ctDNA levels at baseline as well as dynamic assessment of PRD midtreatment and posttreatment might improve risk stratification. To test this hypothesis, we developed a model for dynamic risk stratification in CNSL. First, we performed a random split of the whole study population to obtain a cohort for training (70% of patients) and a separate cohort for validation (30% of patients) that was not used in the model generation process. We selected all variables contained in the IELSG score38 for inclusion in the model. Deep brain lesions as defined in the IELSG score38 were confirmed by central radiologic review. Because detailed information on age and Eastern Cooperative Oncology Group performance status were available for all patients, we included these as continuous rather than binary variables (as in the IELSG score: age <60 years or >60 years; and Eastern Cooperative Oncology Group performance status of 0-1 or 2-4). As new features, because of their predictive power in CNSL, we included the achievement of CR as assessed by MRI (yes/no), ctDNA levels at baseline (log10 haploid genome equivalents [hGE] per mL plasma), and the log10 change of PRD from baseline. Next, we imputed missing values and constructed an ensemble tree-based machine-learning model to predict the possibility of relapse.40 Details on the generation of the final model can be found in supplemental Methods. Finally, we assessed the distribution of relapse probabilities as predicted by the final model, and separated patients into 3 groups: low risk (0%-16.5% relapse probability), intermediate risk (16.5%-50% relapse probability), and high risk (>50% relapse probability).
Statistical analysis and end points
Discrete variables were tabulated and compared using Fisher exact test. Continuous variables were described with their means, medians, and ranges, and compared using the Student t test if a variable was normally distributed or, if not, the Mann-Whitney U test. Survival curves were calculated using the Kaplan-Meier method. Failure-free survival (FFS) was defined as the time from diagnosis to PD, relapse, or lymphoma-associated death, whichever occurred first, and was censored at the time of last available information. Progression-free survival (PFS) was defined as the time from diagnosis to PD, relapse, or death from any cause, whichever occurred first and was censored at the time of last available information. Overall survival (OS) was defined as the time from diagnosis to death from any cause and was censored at the time of last available information. FFS, PFS, and OS were compared between groups using the log-rank test. Univariate analysis was performed using Cox proportional hazards regression. Information on elevated protein levels in the CSF was not available for all patients and, thus, imputed using the R package “mice.” All tests were performed as 2-sided tests. A P value < .05 was considered significant. Statistical analyses were performed using R.41
Results
Patient characteristics
We extracted cell-free DNA from n = 135 plasma and n = 9 CSF samples of n = 58 patients with PCNSL and n = 9 patients with SCNSL. The design of the study is depicted in Figure 1. The median age was 69.6 years (range, 27-85 years), and 33 (49.3%) patients were female (Table 1). Despite being eligible, 5 (7.5%) patients did not receive high-dose MTX–based treatment. Reasons for omitting MTX were early events or patient’s preference. FFS, PFS, and OS of the full study cohort are shown in supplemental Figure 2.
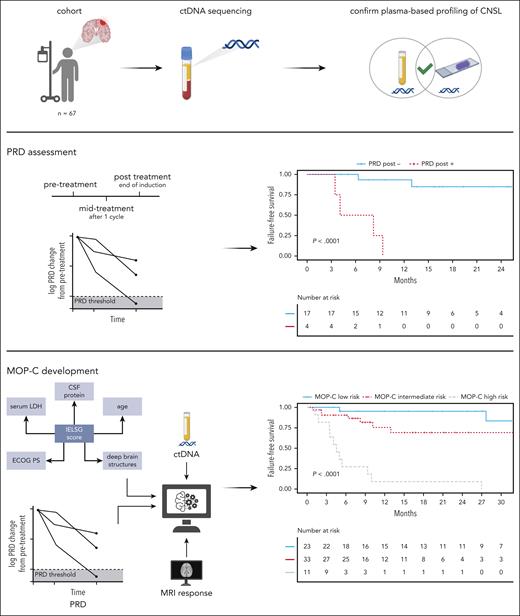
Workflow of the study. Patients (n = 67) with CNSL were identified; ctDNA sequencing was applied to plasma and CSF samples using a targeted gene panel; mutations detected from plasma were confirmed to be CNSL-derived by sequencing of tumor DNA/CSF-derived ctDNA; PRD was developed as a novel biomarker in CNSL and shown to be predictive of outcomes; integration of dynamically assessed clinical and molecular features resulted in development and validation of MOP-C, a tool with high predictive value in CNSL; created with BioRender.com.
Workflow of the study. Patients (n = 67) with CNSL were identified; ctDNA sequencing was applied to plasma and CSF samples using a targeted gene panel; mutations detected from plasma were confirmed to be CNSL-derived by sequencing of tumor DNA/CSF-derived ctDNA; PRD was developed as a novel biomarker in CNSL and shown to be predictive of outcomes; integration of dynamically assessed clinical and molecular features resulted in development and validation of MOP-C, a tool with high predictive value in CNSL; created with BioRender.com.
Patient characteristics
| Patient characteristic . | n or median . | % or range . |
|---|---|---|
| Total patients | 67 | |
| PCNSL | 58 | 68.6 |
| SCNSL | 9 | 13.4 |
| Age (y) | 69.6 | 27-85 |
| Sex | ||
| Female | 33 | 49.3 |
| Male | 34 | 50.7 |
| ECOG PS ≥ 2 | 27 | 40.3 |
| IELSG risk group | ||
| Low | 7 | 10.4 |
| Intermediate | 41 | 61.2 |
| High | 19 | 28.4 |
| Treatment | ||
| MATRix/MARTA | 34 | 50.7 |
| PRIMAIN | 22 | 32.8 |
| Other | 11 | 16.4 |
| HCT-ASCT | 29 | 43.3 |
| Consolidative WBRT | 7 | 10.4 |
| Relapse | 20 | 29.9 |
| Death | 25 | 37.3 |
| Follow-up survivors (mo) | 17.5 | 4.1-54 |
| Patient characteristic . | n or median . | % or range . |
|---|---|---|
| Total patients | 67 | |
| PCNSL | 58 | 68.6 |
| SCNSL | 9 | 13.4 |
| Age (y) | 69.6 | 27-85 |
| Sex | ||
| Female | 33 | 49.3 |
| Male | 34 | 50.7 |
| ECOG PS ≥ 2 | 27 | 40.3 |
| IELSG risk group | ||
| Low | 7 | 10.4 |
| Intermediate | 41 | 61.2 |
| High | 19 | 28.4 |
| Treatment | ||
| MATRix/MARTA | 34 | 50.7 |
| PRIMAIN | 22 | 32.8 |
| Other | 11 | 16.4 |
| HCT-ASCT | 29 | 43.3 |
| Consolidative WBRT | 7 | 10.4 |
| Relapse | 20 | 29.9 |
| Death | 25 | 37.3 |
| Follow-up survivors (mo) | 17.5 | 4.1-54 |
ECOG PS, Eastern Cooperative Oncology Group performance status; HCT-ASCT, high-dose chemotherapy followed by autologous stem cell transplantation; MARTA, methotrexate, cytarabine, and rituximab; MATRix, methotrexate, cytarabine, thiotepa, and rituximab; PRIMAIN, procarbazine, methotrexate, and rituximab; WBRT, whole-brain radiotherapy.
ctDNA
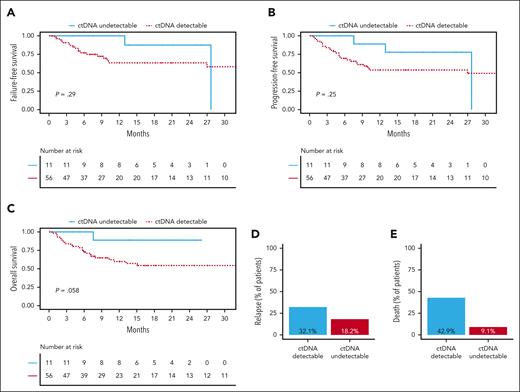
Total cell-free DNA concentrations were particularly low in CSF samples (median, 1.97; range, 0.5-79 ng/mL) as compared with plasma samples (median, 13.68; range, 0.9-208.5 ng/mL). In contrast, ctDNA levels (the share of total cell-free DNA originating from the tumor) were increased in CSF samples (median, 2.18; range, 0-3.9 log hGE per mL) as compared with plasma samples (median, 1.49; range, 0-3 log hGE per mL; supplemental Figure 3A-B). No association was observed between the tumor volume at baseline (supplemental Figure 3C) or clinical characteristics and plasma ctDNA concentrations at baseline (supplemental Figure 3D-K). Of note, elevated CSF protein levels were associated with higher CSF ctDNA concentrations (supplemental Figure 3J). Interestingly, most patients with undetectable plasma ctDNA at baseline remained relapse-free and alive throughout the observation period (ctDNA undetectable vs ctDNA detectable: 2-year FFS: 87.5% vs 63.4% [P = .2900]; 2-year PFS: 77.8% vs 53.6% [P = .2500]; 2-year OS: 88.9% vs 54.4% [P = .0580]; Figure 2). Of note, statistical significance was not reached, presumably because of the low number of patients with undetectable ctDNA.
Undetectable ctDNA at baseline correlates with favorable outcomes in patients with CNSL. (A) Comparison of FFS between patients with undetectable ctDNA at baseline (blue solid line) and patients with detectable ctDNA at baseline (red dashed line; n = 67 patients). (B) Comparison of PFS between patients with undetectable ctDNA at baseline (blue solid line) and patients with detectable ctDNA at baseline (red dashed line; n = 67 patients). (C) Comparison of OS between patients with undetectable ctDNA at baseline (blue solid line) and patients with detectable ctDNA at baseline (red dashed line; n = 67 patients). (D) Bar plot comparing the fraction of patients suffering from relapse between patients with detectable ctDNA at baseline (blue) and patients with undetectable ctDNA at baseline (red). (E) Bar plot comparing the fraction of patients that died between patients with detectable ctDNA at baseline (blue) and patients with undetectable ctDNA at baseline (red). Statistical significance was assessed using log-rank tests.
Undetectable ctDNA at baseline correlates with favorable outcomes in patients with CNSL. (A) Comparison of FFS between patients with undetectable ctDNA at baseline (blue solid line) and patients with detectable ctDNA at baseline (red dashed line; n = 67 patients). (B) Comparison of PFS between patients with undetectable ctDNA at baseline (blue solid line) and patients with detectable ctDNA at baseline (red dashed line; n = 67 patients). (C) Comparison of OS between patients with undetectable ctDNA at baseline (blue solid line) and patients with detectable ctDNA at baseline (red dashed line; n = 67 patients). (D) Bar plot comparing the fraction of patients suffering from relapse between patients with detectable ctDNA at baseline (blue) and patients with undetectable ctDNA at baseline (red). (E) Bar plot comparing the fraction of patients that died between patients with detectable ctDNA at baseline (blue) and patients with undetectable ctDNA at baseline (red). Statistical significance was assessed using log-rank tests.
Plasma ctDNA sequencing reveals detection of CNSL-derived mutational profiles
In total, we were able to detect n = 1295 mutations in plasma samples of patients with CNSL at baseline. To assess whether the mutational profiles derived from plasma ctDNA sequencing are truly representative of CNSL, we additionally performed targeted gene panel sequencing of tumor DNA obtained from stereotactic brain biopsies available for a subset of patients (see “Methods” for details). The mutational profiles obtained by tumor DNA sequencing were representative of CNSL, including mutations in key genes involved in CNSL pathogenesis, such as MYD88, PIM1, CD79B, and PCLO (supplemental Figure 4A). A similar picture emerged when assessing the mutational profiles of CSF-derived ctDNA (supplemental Figure 4B). Overall, we were able to detect mutations in 7 of 9 (77.8%) CSF samples. Next, we compared plasma ctDNA-derived mutations in patients with available plasma-derived ctDNA and either CSF-derived ctDNA or tumor DNA with the mutational profiles obtained from CSF-derived ctDNA or tumor DNA. Driver mutations (MYD88, PIM1, CD79B, and PCLO), mutations affecting the coding genome, and noncoding mutations were detected in plasma-derived ctDNA in 19 of 67 (28.4%), 48 of 67 (71.6%), and 56 of 67 (83.6%) patients, respectively (supplemental Figure 4C). In line with our previous experience with systemic lymphoma,37 we were able to reidentify 97 of 171 (56.7%) and 94 of 127 (74.0%) mutations detected in plasma-derived ctDNA in the corresponding CSF and tumor samples, respectively (supplemental Figure 4D). In particular, 19 of 53 (35.8%; CSF-derived ctDNA) and 37 of 58 (63.8%; tumor DNA) of mutations affecting the coding genome as well as 78 of 118 (66.1%; CSF-derived ctDNA) and 57 of 69 (82.6%; tumor DNA) of noncoding mutations detected in plasma-derived ctDNA were present in the corresponding CSF and tumor samples (supplemental Figure 4E-F).
PRD
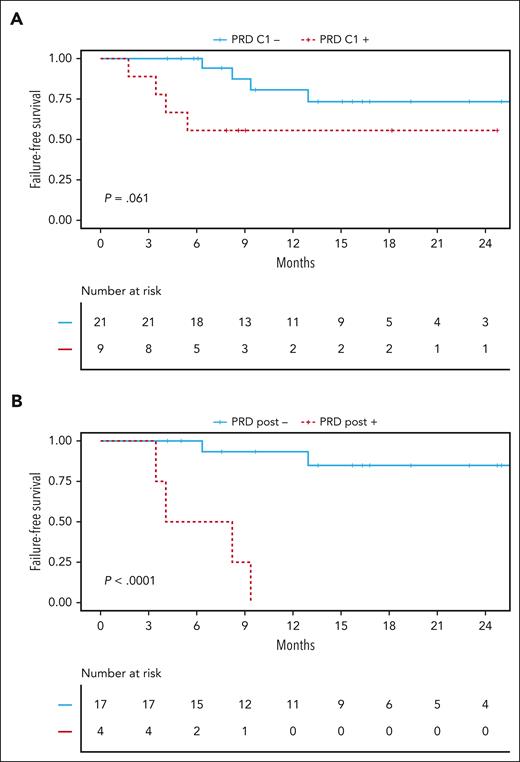
To overcome the limited power of MRI for the detection of true CR in CNSL, we tracked mutations in plasma samples after 1 cycle (midtreatment) and at the end of induction treatment (posttreatment). Because our approach does not rely on previous mutational profiling of DNA derived from tumor tissue, we called it PRD. In addition to the detection of 447 of 1295 (34.5%) mutations affecting the coding genome at baseline, our targeted gene panel allowed for robust detection of targets in noncoding regions susceptible to somatic hypermutation (supplemental Figure 4G).42-44 These targets enhanced ultrasensitive detection of PRD even when other mutations were only sparsely detected in a sample. At this point in the study, 30% of the whole study cohort were randomly selected and withheld for validation, whereas the remaining 70% were used for model training (supplemental Figure 5). Patients having no quantifiable ctDNA at baseline were considered to have PRD-negative status. To define the limit of negativity (blank), cell-free DNA from 10 healthy donors without any prior history of cancer were used as negative PRD samples. PRD was calculated in each of these 10 healthy donor cell-free DNA samples based on identified mutations in 8 baseline ctDNA samples from this study, resulting in 80 independent assessments of PRD. The limit of negativity (blank) was calculated as previously reported.37 PRD was defined to be negative if a negative change of ≥2 log10 levels from baseline was achieved. Midtreatment persistence of PRD showed a trend toward impaired 2-year FFS (55.6% vs 73.3%; P = .0610; Figure 3A). Strikingly, posttreatment persistence of PRD showed the increasing value of its assessment over the course of the treatment with 2-year FFS rates of 0% vs 84.9% (P < .0001) for patients with PRD-positive and those with PRD-negative statuses, respectively, directly after treatment (Figure 3B).
Persistence of PRD is a risk factor in CNSL. (A) Comparison of FFS between patients with PRD-negative status (blue solid line) and patients with PRD-positive status (red dashed line) after 1 cycle of treatment within all patients of the training cohort with available follow-up samples (n = 30 patients). (B) Comparison of FFS comparing patients with PRD-negative status (blue solid line) and those with PRD-positive status (red dashed line) after completion of induction treatment within all patients of the training cohort with available follow-up samples (n = 21 patients). Statistical significance was assessed using log-rank tests.
Persistence of PRD is a risk factor in CNSL. (A) Comparison of FFS between patients with PRD-negative status (blue solid line) and patients with PRD-positive status (red dashed line) after 1 cycle of treatment within all patients of the training cohort with available follow-up samples (n = 30 patients). (B) Comparison of FFS comparing patients with PRD-negative status (blue solid line) and those with PRD-positive status (red dashed line) after completion of induction treatment within all patients of the training cohort with available follow-up samples (n = 21 patients). Statistical significance was assessed using log-rank tests.
Metagenomic analysis of cell-free DNA can help to distinguish between persistent disease and infections
Because repeated CNS biopsies are often not feasible, histopathological assessment is frequently omitted in relapsed/refractory CNSL. However, structural alterations of the CNS caused by infections are sometimes difficult to discern from CNSL and, thus, require an additional invasive biopsy. In a patient of this study, CNSL relapse was suspected but repeated invasive biopsy revealed cerebral toxoplasmosis instead of vital tumor tissue. We assessed plasma- and CSF-derived ctDNA obtained at the time of suspected relapse but were unable to detect any mutations despite de novo analysis. In addition to human-derived DNA, the cell-free DNA pool contains DNA derived from different pathogens such as bacteria, viruses, and protozoa. Applying a metagenomic approach (see supplemental Methods for details), we were able to detect Toxoplasma gondii at high levels in both plasma- and CSF-derived ctDNA (supplemental Figure 6).
Molecular prognostic index for CNSL (MOP-C)
Finally, we developed a tool for outcome prediction in CNSL (see “Methods” for details). The final model was named MOP-C: molecular prognostic index for CNSL. Upon entering the required variables, MOP-C returns a value between 0% and 100%, which indicates an individual patient’s relapse probability. Based on the distribution of MOP-C scores in the training cohort, we divided patients into 3 distinct risk groups: low risk (0%-16.5%), intermediate risk (16.5%-50%), and high risk (>50%; Figure 4A). MOP-C was highly accurate in predicting relapses, which is highlighted by correct identification of relapse in all patients in the high-risk group both in the training and validation cohort. In general, the prognostic value of MOP-C was not only evident in the training cohort but equally present in the validation cohort (Figure 4B). Consequently, risk stratification by MOP-C was highly predictive of FFS in the training cohort (2-year FFS low risk: 100% vs intermediate risk: 70.5% vs high risk: 0%; P < .0001 [low risk vs high risk]; Figure 4C), the validation cohort (2-year FFS low risk: 83.3% vs intermediate risk: 70% vs high risk: 33.3%; P = .0450 [low risk vs high risk]; Figure 4D), and the whole study cohort (2-year FFS low risk: 95.2% vs intermediate risk: 69.1% vs high risk: 9.1%; P < .0001 [low risk vs high risk]; Figure 4E). Of note, a sensitivity analysis revealed the prognostic value of MOP-C to be preserved when excluding patients not receiving MTX-based treatment (supplemental Figure 7A) or patients with SCNSL (supplemental Figure 7B). When comparing the predictive value of MOP-C with the IELSG score, MOP-C revealed substantially improved performance with regard to FFS (hazard ratio [HR] per MOP-C risk group, 6.60; 95% confidence interval [CI], 3.12-13.97; P < .0001 vs HR per IELSG risk group, 2.64; 95% CI, 1.21-5.76; P = .0147; Figure 5A), PFS (HR per MOP-C risk group, 3.24; 95% CI, 1.85-5.68; P < .0001 vs HR per IELSG risk group, 2.44; 95% CI, 1.28-4.66; P = .0067), and OS (HR per MOP-C risk group, 2.73; 95% CI, 1.54-4.85; P = .0006 vs HR per IELSG risk group, 2.37; 95% CI, 1.19-4.75; P = .0146). The improvement observed here is mainly attributable to a refined separation of the intermediate- and high-risk IELSG risk groups by MOP-C (Figure 5B-C).
MOP-C is predictive of outcomes in patients with CNSL. (A) Density plot depicting the distribution of MOP-C scores (0-1) within the training cohort. x-axis depicts MOP-C score; y-axis depicts density. Dashed lines indicate 2 density peaks (0.165; 0.5) used for the development of 3 separate risk groups: low risk ≤ 0.165; intermediate risk = 0.165-0.5; high risk ≥ 0.5. (B) Dot plot depicting MOP-C scores, comparing patients with and without relapse in the training and validation cohort. Each dot represents a single patient, dashed lines indicate the cut-offs between risk groups, color codes depict different risk groups, and numbers depict the percentage of patients with relapse within every risk group. (C) Comparison of FFS between patients with low-risk MOP-C scores (blue solid line), intermediate-risk MOP-C scores (red dashed line), and those with high-risk MOP-C scores (gray dashed line) in the training cohort (n = 48 patients). (D) Comparison of FFS between patients with low-risk MOP-C scores (blue solid line), patients with intermediate-risk MOP-C scores (red dashed line), and those with high-risk MOP-C scores (gray dashed line) in the validation cohort (n = 19 patients). (E) Comparison of FFS between patients with low-risk MOP-C scores (blue solid line), intermediate-risk MOP-C scores (red dashed line), and those with high-risk MOP-C scores (gray dashed line) in the whole study cohort (n = 67 patients). Statistical significance was assessed using log-rank tests (high risk vs low risk).
MOP-C is predictive of outcomes in patients with CNSL. (A) Density plot depicting the distribution of MOP-C scores (0-1) within the training cohort. x-axis depicts MOP-C score; y-axis depicts density. Dashed lines indicate 2 density peaks (0.165; 0.5) used for the development of 3 separate risk groups: low risk ≤ 0.165; intermediate risk = 0.165-0.5; high risk ≥ 0.5. (B) Dot plot depicting MOP-C scores, comparing patients with and without relapse in the training and validation cohort. Each dot represents a single patient, dashed lines indicate the cut-offs between risk groups, color codes depict different risk groups, and numbers depict the percentage of patients with relapse within every risk group. (C) Comparison of FFS between patients with low-risk MOP-C scores (blue solid line), intermediate-risk MOP-C scores (red dashed line), and those with high-risk MOP-C scores (gray dashed line) in the training cohort (n = 48 patients). (D) Comparison of FFS between patients with low-risk MOP-C scores (blue solid line), patients with intermediate-risk MOP-C scores (red dashed line), and those with high-risk MOP-C scores (gray dashed line) in the validation cohort (n = 19 patients). (E) Comparison of FFS between patients with low-risk MOP-C scores (blue solid line), intermediate-risk MOP-C scores (red dashed line), and those with high-risk MOP-C scores (gray dashed line) in the whole study cohort (n = 67 patients). Statistical significance was assessed using log-rank tests (high risk vs low risk).
MOP-C improves current risk stratification strategies in CNSL. (A) Univariate analysis of the IELSG and MOP-C risk groups regarding FFS. HRs and 95% CIs are expressed per risk group (intermediate vs low risk, and high vs intermediate risk), respectively. Statistical significance was assessed using Cox regression analysis (n = 67 patients). (B) Sankey plot depicting patient flows from IELSG risk groups to MOP-C risk groups. Color code depicts different risk groups, and size of transition flows indicates number of patients. (C) Dot plot depicting MOP-C risk groups within the 3 IELSG risk groups, comparing patients with and without relapse. Each dot represents a single patient, dashed lines indicate the cut-offs between risk groups, color code depicts different MOP-C risk groups, and numbers depict the percentage of patients with relapse within every risk group.
MOP-C improves current risk stratification strategies in CNSL. (A) Univariate analysis of the IELSG and MOP-C risk groups regarding FFS. HRs and 95% CIs are expressed per risk group (intermediate vs low risk, and high vs intermediate risk), respectively. Statistical significance was assessed using Cox regression analysis (n = 67 patients). (B) Sankey plot depicting patient flows from IELSG risk groups to MOP-C risk groups. Color code depicts different risk groups, and size of transition flows indicates number of patients. (C) Dot plot depicting MOP-C risk groups within the 3 IELSG risk groups, comparing patients with and without relapse. Each dot represents a single patient, dashed lines indicate the cut-offs between risk groups, color code depicts different MOP-C risk groups, and numbers depict the percentage of patients with relapse within every risk group.
Discussion
Here, we present a novel, dynamic, and entirely noninvasive risk profiling strategy in CNSL. Our approach is based on tumor-agnostic plasma ctDNA sequencing and assessment of PRD. In this context, tumor-agnostic means that our approach does not require prior genotyping information from a tumor biopsy but independently identifies CNSL-derived mutations in plasma-derived ctDNA and makes them trackable in subsequent samples obtained during the course of the disease. The major findings were that (1) tumor-agnostic plasma ctDNA sequencing in CNSL is feasible; (2) the absence of ctDNA from plasma is associated with a low risk of relapse; (3) PRD is a novel biomarker and highly predictive of outcomes in CNSL, especially when assessed posttreatment; and (4) we successfully developed and validated MOP-C, a novel tool that reliably predicts relapses and compares favorably with current state-of-the-art risk stratification and response evaluation in CNSL.
PCNSL harbors recurrent mutations in several genes such as MYD88, PIM1, CD79B, TBL1XR1, CARD11, PCLO, IGLL5, and PRDM1.15-18,42,43,45-51 In addition to tissue-based genotyping, subsequent tracking of detected mutations in CSF by digital droplet polymerase chain reaction has successfully been explored.52,53 To date, studies evaluating the feasibility of plasma-derived ctDNA sequencing in CNSL mainly focused on MYD88 using either digital droplet polymerase chain reaction, high-throughput sequencing, or both but generally reported low discovery rates.28,29,54 Recently, Mutter et al. presented the first study successfully detecting several genetic aberrations associated with CNSL by performing tumor-informed plasma ctDNA sequencing.36 Additionally, the study provided a proof of principle for tumor-agnostic genotyping in CNSL using CSF and finally resulted in the development of a statistical model for noninvasive differentiation between CNSL and other primary brain tumors. In contrast, our ultrasensitive plasma ctDNA sequencing approach allowed for the detection of CNSL-associated mutations without the necessity of prior tumor DNA sequencing. Although reliable detection of functionally relevant genes was only achieved in a subset of patients, which is in line with the results presented by Mutter et al.,36 we were able to detect ctDNA in 83.6% of patients. Whether the presence or absence of ctDNA in the plasma of patients with CNSL is a result of different degrees of compromise of the blood–brain barrier, simply a function of tumor burden, or a biomarker for occult peripheral disease remains elusive. Of note, there was no correlation between tumor volumes and plasma-derived ctDNA levels in our cohort. In line with previous results,36 only 2 of 11 patients with undetectable plasma ctDNA at baseline experienced relapse.
Our approach for the detection of PRD also made use of mutations located in hypermutated noncoding regions. Somatic hypermutation is a hallmark of CNSL pathogenesis and targets oncogenes such as PIM1, PAX5, MYC, and KLHL14.17,18 It was recently reported that aberrant somatic hypermutations might be enhanced in PCNSL compared with other lymphomas.43 The large number of such mutations detected by our approach made the assessment of PRD feasible in our study cohort. Encouraged by previous results in DLBCL,26,35,55 we chose an equal threshold for PRD negativity of a 2 log–level reduction (100-fold lower PRD than at baseline), resulting in a strong ability to predict outcomes in CNSL. Of note, midtreatment assessment of PRD resulted in a poorer separation of risk groups than posttreatment assessment of PRD. This fact might be explained by a slightly prolonged time to response than in DLBCL, in which achievement of very early molecular responses is closely correlated with favorable outcomes.35 Overall, the main advantage of PRD-based response evaluation might be attributable to early unmasking of true CR, a task that MRI often fails. Therefore, the achievement of molecular CR as assessed by PRD might allow for premature end of treatment in case of severe toxicities. It might be advisable to repeat assessments of PRD over time (e.g. every 3 months) because these patients could be at higher risk of relapse. Of note, the inclusion of additional noncoding genomic regions that have recently been shown to be subject to highly frequent mutations56 into a revised target region for our approach might further enhance the sensitivity of PRD by increasing the number of available individual tumor-specific genetic targets to track over the course of treatment. Importantly, PRD can be measured in a batch of multiple samples on larger sequencers or in single samples on small-scale sequencers, allowing for the assessment of single patients within ∼7 days. Removing genes that were rarely affected by mutations in this study from the target region might further increase the use of smaller sequencers in future studies.
Finally, we integrated our findings with established clinical risk factors and developed MOP-C. MOP-C improved outcome prediction as compared with the IELSG risk score in our study.38 Although the IELSG score has proven to be a valuable tool for risk stratification in CNSL and has been broadly applied ever since, it has two major limitations. First, all variables included in the score are used in binary form and with equal weight; however, the assignment of continuous variables might result in improved precision. Second, it does not correct for molecular or radiographic responses achieved during and after treatment, factors that are becoming increasingly valuable for outcome prediction in various lymphomas including CNSL.35-37,55,57 Of note, the incorporation of these dynamic features differentiates MOP-C from other prognostic indices in which the calculation is usually completed at baseline. The additional value of MOP-C is especially highlighted by its ability to further subdivide patients of the IELSG intermediate- and high-risk groups into patients at low and high risk of relapse.
Current efforts in refining the treatment of CNSL mainly focus on improving the risk-to-benefit ratio in individual patients. The goal is to maintain sufficient lymphoma control while reducing potentially life-threatening side effects. For instance, the OptiMATe trial (ClinicalTrials.gov identifier: NCT04931368) aims to improve event-free survival with a reduced intensity induction. In this context, MOP-C may provide a valuable resource to identify patients with insufficient responses to reduced intensity induction. Switching to higher intensity treatments for patients at high risk only based on MOP-C could preserve the reduced toxicity for most patients while maintaining overall safety through tight PRD monitoring and use of MOP-C.
The presented approach has several limitations. In line with previous results,36 the identification of potential biomarkers for targeted therapies penetrating the CNS (e.g. MYD88 hot-spot mutations for treatment with Bruton's tyrosine kinase inhibitors) cannot be provided for every patient using only plasma-derived ctDNA and might, thus, favor sequencing of tumor DNA. Furthermore, with only a small number of patients with SCNSL assessed, additional studies are required to determine whether these findings are entirely transferable to SCNSL. Finally, clinical trials are warranted for prospective validation of PRD and MOP-C. The dynamic assessment of all variables included in the IELSG score as well as CSF-derived ctDNA might provide additional prognostic value.
Many of these current shortcomings will be addressed in the upcoming MTR2 trial (ClinicalTrials.gov identifier: NCT05583071) in which PRD and MOP-C will be assessed prospectively. In this study, we will also further standardize our assay procedure, for example, by using specialized cell-free DNA collection tubes to minimize any risk of genomic DNA release into plasma between sample collection and processing that might, at least theoretically, reduce the sensitivity of PRD assessment. After this validation, PRD monitoring might be used for future clinical trials, aiming at early therapeutic interventions before deterioration of the patient's general condition because of overt CNSL relapse. MOP-C might be applied to evaluate treatment de-escalation in patients at low risk, different consolidation strategies in patients at low risk vs those at high risk, or add-on designs with new compounds such as bispecific antibodies and chimeric antigen receptor T-cell therapy in patients at high risk.
In summary, our results suggest that MOP-C has the potential to refine current state-of-the-art risk stratification and response evaluation, which might improve treatment guidance and, ultimately, outcomes of patients with CNSL.
Acknowledgments
The authors thank Elisabeth Kirst for her excellent technical assistance. Furthermore, the authors thank the reviewers for their thoughtful and thorough peer-review. High-throughput sequencing analyses were carried out at the production site West German Genome Center Cologne.
J.-M.H. was supported by a MD Research Stipend of the Else Kröner Forschungskolleg Clonal Evolution in Cancer, University Hospital Cologne, Cologne, Germany. J.M. was supported by a MD Research Stipend of the Köln Fortune program, University of Cologne, Cologne, Germany. R.F.S. is a professor at the Cancer Research Center Cologne Essen, funded by the Ministry of Culture and Science of the State of North Rhine-Westphalia. This work was partially funded by the German Ministry for Education and Research as BIFOLD - Berlin Institute for the Foundations of Learning and Data (references 01IS18025A and 01IS18037A). This work was supported by the Deutsche Forschungsgemeinschaft Research Infrastructure West German Genome Center (407493903) as part of the Next Generation Sequencing Competence Network (project 423957469). The research was partly funded by the Deutsche Forschungsgemeinschaft (RE 2246/13-1 and SFB1399-A01 SFB-Geschäftszeichen 455784452 [H.C.R.], BO5316/3-1 [S.B.], BO5316/2-1 [S.B.], TR1268/3-1 [B.v.T.], and SFB1530 [S.B.]) as well as grants by the Frauke Weiskam and Christel Ruranski Foundation, and the Else Kröner Fresenius Stiftung (Kolleg 2016).
Authorship
Contribution: J.-M.H., B.v.T., and S.B. developed the study design; J.-M.H., J.M., J.S., P.G., H.B.-W., N.K., R.L., F.U., J.W., and B.v.T. were responsible for sample collection, processing, and storage; J.-M.H., J.M., P.G., N.S., F.U., and R.L. analyzed the clinical data; J.-M.H., J.M., J.S., K.B., and S.B. performed bioinformatic and downstream analyses; J.-M.H., T.B.-M., R.F.S., and S.B. developed MOP-C; T.B.-M. and R.F.S. created the interface for usage of MOP-C; D.R. and M.I.R. analyzed tumor volumes; M.M.-R., M.D., F.U., T.B., H.C.R., M.H., P.B., and B.v.T. recruited patients and provided material and resources; J.-M.H., B.v.T., and S.B. wrote the manuscript; and all authors revised and agreed to the final version of the manuscript.
Conflict-of-interest disclosure: J.-M.H. is an adviser or consultant for Miltenyi, Genmab, Incyte, Swedish Orphan Biovitrum, SERB Pharmaceuticals, and Novartis; reports research funding from Incyte (to institution), and Novartis (to institution); and reports travel support from Swedish Orphan Biovitrum, SERB Pharmaceuticals, and Novartis. F.U. has received honoraria from Roche; and reports travel support from AbbVie, Incyte, Gilead Kite, Janssen-Cilag, and Swedish Orphan Biovitrum. N.K. is an advisor or consultant for AstraZeneca; has received honoraria from AbbVie, AstraZeneca, BMS, and Roche; reports research funding from AstraZeneca (to institution), and Gilead (to institution); and reports travel support from AbbVie, AstraZeneca, BeiGene, Celgene, Gilead, and Janssen. P.B. is an adviser or consultant for Takeda, Bristol Myers Squibb, Merck Sharp & Dohme, Roche, Novartis, and Amgen; has received honoraria from Takeda, Novartis, Bristol Myers Squibb, Roche, Merck Sharp & Dohme, Gilead Kite, and Incyte; and reports research funding from Takeda (to institution), MPI (to institution), Roche (to institution), Novartis (to institution), Merck Sharp & Dohme (to institution), and Amgen (to institution). B.v.T. is an adviser or consultant for Allogene, Bristol Myers Squibb/Celgene, Cerus, Incyte, IQVIA, Lilly, Miltenyi, Novartis, Noscendo, Pentixapharm, Roche, Amgen, Pfizer, Takeda, Merck Sharp & Dohme, Swedisch Orphan Biovitrum, Qualworld, Pierre Fabre, and Gilead Kite; has received honoraria from AbbVie, AstraZeneca, Bristol Myers Squibb, Gilead Kite, Incyte, Lilly, Novartis, Roche Pharma AG, Takeda, and Merck Sharp & Dohme; reports research funding from Esteve (to institution), Novartis (to institution), Merck Sharp & Dohme (to institution), and Takeda (to institution); and reports travel support from AbbVie, AstraZeneca, Gilead Kite, Lilly, Merck Sharp & Dohme, Pierre Fabre, Roche, Takeda, and Novartis. S.B. is a consultant for Galapagos; has received honoraria from Takeda; reports travel support from Takeda; and reports being founder and shareholder of Liqomics. The remaining authors declare no competing financial interests.
The current affiliation for M.D. is Institute of Neuropathology, University Hospital Düsseldorf and Medical Faculty, Heinrich Heine University Düsseldorf, Düsseldorf, Germany.
Correspondence: Jan-Michel Heger, Department I of Internal Medicine, University Hospital Cologne, Weyertal 115c, 50931 Cologne, Germany; email: jan-michel.heger@uk-koeln.de; and Sven Borchmann, Department I of Internal Medicine, University Hospital Cologne, Weyertal 115c, 50931 Cologne, Germany; email: sven.borchmann@uk-koeln.de.
References
Author notes
∗B.v.T. and S.B. contributed equally to this study.
Primary sequencing data cannot be deposited publicly because of legal requirements and patient data protection. Upon entering a collaboration agreement, and in consultation with the local ethics committee, access to primary sequencing data might be granted.
All secondary data derived from primary sequencing data are available within the article, supplemental Information, or supplemental Data files.
Original code and further information on resources and reagents are available on request from the corresponding authors, Jan-Michel Heger (jan-michel.heger@uk-koeln.de) and Sven Borchmann (sven.borchmann@uk-koeln.de).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal