A new TCR-mimic monoclonal antibody specific for the WT1-derived epitope RMF presented by HLA-A2, ESK2, was developed.
ESK2 was engineered into a new CAR T format, AbTCR-CSR, which comprises primary and CSR, directed against 2 antigens, WT1 and CD33 in AML.
Visual Abstract
Chimeric antigen receptor T-cell (CAR T) therapy has produced remarkable clinical responses in B-cell neoplasms. However, many challenges limit this class of agents for the treatment of other cancer types, in particular the lack of tumor-selective antigens for solid tumors and other hematological malignancies, such as acute myeloid leukemia (AML), which may be addressed without significant risk of severe toxicities while providing sufficient abundance for efficient tumor suppression. One approach to overcome this hurdle is dual targeting by an antibody–T-cell receptor (AbTCR) and a chimeric costimulatory signaling receptor (CSR) to 2 different antigens, in which both antigens are found together on the cancer cells but not together on normal cells. To explore this proof of concept in AML, we engineered a new T-cell format targeting Wilms tumor 1 protein (WT1) and CD33; both are highly expressed on most AML cells. Using an AbTCR comprising a newly developed TCR-mimic monoclonal antibody against the WT1 RMFPNAPYL (RMF) epitope/HLA-A2 complex, ESK2, and a secondary CSR comprising a single-chain variable fragment directed to CD33 linked to a truncated CD28 costimulatory fragment, this unique platform confers specific T-cell cytotoxicity to the AML cells while sparing healthy hematopoietic cells, including CD33+ myelomonocytic normal cells. These data suggest that this new platform, named AbTCR-CSR, through the combination of a AbTCR CAR and CSR could be an effective strategy to reduce toxicity and improve specificity and clinical outcomes in adoptive T-cell therapy in AML.
Introduction
Chimeric antigen receptor (CAR) T-cell therapy has achieved remarkable clinical efficacy in B-cell hematological malignancies.1,2 However, CAR T cells also elicit toxicities, including cytokine release syndrome, neurologic toxicity, and on-target/off-tumor recognition.3 Current CAR constructs include an antigen-binding domain, usually a single-chain variable fragment (scFv) derived from a monoclonal antibody that recognizes antigen on tumor cells, which is linked to an intracellular signaling domain derived from CD3ζ, a component of the T-cell receptor (TCR) complex, as well as a costimulatory domain that often comprises a region of at least 1 costimulatory molecule such as CD28 or 4-1BB. Direct fusion of the antigen-recognition domain to the downstream T-cell activation domain from CD3ζ, however, can create an exaggerated synthetic activation signal that results in excessive T-cell activation and cytokine release as well as early exhaustion of T cells.3
Additional specificity challenges further limit the traditional class of CAR T-cell agents for the treatment of cancer types other than B-cell neoplasms in that there is a paucity of tumor-specific antigens for solid tumors and myeloid leukemias, such as AML, that may be addressed without significant risk of life-threatening normal cell killing.4,5 Identification of ideal target antigens that are ubiquitously expressed on all tumor cells but are only minimally expressed on normal tissues is a major challenge. In AML, myeloid lineage antigens such as CD33, CD123, and others are shared between leukemia blasts and normal myeloid cells, which could lead to severe myelosuppression after effective CAR T-cell therapy. In contrast, intracellular tumor antigens that may be more tumor-specific have been infrequently explored in CAR T-cell therapy.6 We previously described the therapeutic efficacy in mouse models of a human TCR-mimic (TCRm) monoclonal antibody (mAb), ESK1, specific for the Wilms tumor protein (WT1)-derived RMFPNAPYL (RMF) peptide in the context of HLA-A0201 molecules.7,8 WT1 oncoprotein is an intracellular, oncogenic transcription factor that is selectively overexpressed in a wide range of leukemias9 and solid cancers.10 RMF is a well-validated epitope of WT1 that has been explored preclinically and clinically, including peptide or dendritic cell vaccinations, and adoptive T-cell therapy.11-15 Preclinical studies using CAR T-cell and bispecific antibody formats have also been explored.16,17 Because the original RMF/HLA-A2–specific TCRm, ESK1, displayed off-target reactivity,18,19 we now report the development of a second-generation TCRm specific to the RMF/HLA-A2 complex, named ESK2, which exhibited improved target specificity in vitro.
To overcome the limitations of CAR T cells, we designed a different platform in which an antibody TCR (AbTCR), fusing the antigen-binding fragment (Fab) of a primary antigen–targeting antibody to a γδTCR-signaling domain to facilitate more natural TCR/CD3 signaling,20 is paired with a second costimulatory signaling receptor (CSR) reactive with a second different antigen. The AbTCR γδTCR signaling yields lower cytokine release and reduced T-cell exhaustion while maintaining comparable tumor inhibition in a patient-derived xenograft model.21
To further improve the target specificity and potency in AML, we generated a new version of this cell platform (termed ARTEMIS 2.0; in this paper referred to as AbTCR-CSR), directed to 2 highly expressed antigens in AML: WT1 and CD33. The new platform contains an ESK2 AbTCR against WT1 and an anti-CD33 chimeric CSR consisting of an anti-CD33 scFv linked to the signaling domain of the costimulatory molecule CD28. Because neither antigen is completely leukemia specific, the requirement for both receptors to bind for optimal killing makes this approach more specific. Although the recognition of the CSR to its target antigen CD33 alone did not deplete the target cells, it enhanced the cytolytic activity of the primary receptor to RMF/HLA-A∗02:01, thus reducing the concern of on-target/off-tumor toxicity that hampers CD33 CAR T-cell therapies due to the toxicity against CD33-expressing normal myeloid lineage cells. We demonstrated potent and specific cytotoxicity of ESK2-AbTCR-CSR–engineered T cells only against the leukemia targets that are both WT1/HLA-A2+ and CD33+, both in vitro and in xenograft mouse models. No significant killing by the AbTCR-CSR cells was observed against normal blood cell subsets that are CD33+. T cells coexpressing anti-CD33 CSR and an irrelevant primary AbTCR did not kill WT1+/HLA-A2+/CD33+ AML cells, further verifying that antitumor activity required the primary recognition signal in addition to CD33, thereby providing additional specificity.
Materials and methods
Selection and characterization of scFv specific to WT1 RMF peptide/HLA-A2 complexes
Biologically active antibody constructs were selected from Eureka Therapeutics’ E-ALPHA phage library, a collection of human scFv antibody phage display libraries containing >10 × 1010 unique clones. The 14 ESK2 clones with unique sequences (and sequences different from ESK1) were chosen based on their selectivity profiles and characterization in various assays; their antigen-recognition domains were engineered into AbTCR constructs (details are in the supplemental Materials, available on the Blood website).
Generation of AbTCR and AbTCR-CSR constructs and characterization
Nucleic acid sequences from the 14 ESK2 clones were engineered to encode anti-WT1 AbTCR alone (the ESK2 AbTCR constructs) or both anti-WT1 AbTCR and anti-CD33 CSR constructs (ESK2 AbTCR-CSR). Vectors encoding the ESK2 AbTCR constructs were used to transduce primary T cells. Eight of 14 different ESK2 AbTCR T cells were selected based on their cytotoxicity against RMF-loaded T2 cells and low cross-reactivity with other peptides. These 8 ESK2 AbTCR T cells were further narrowed down to 4 using stable target cell line targets that were generated by ectopically expressing the RMF peptide region (RMF minigene) in HLA-A∗02:01–positive cell line SK-HEP1. Finally, the specificity was tested using the AML cell lines SKM-1 and SET-2, which are WT1 and HLA-A2 positive, respectively. Two of 4 ESK2 AbTCRs, which were derived from ESK2 antibody clones no. 18 and no. 34, were selected for generation of AbTCR-CSR T cells, which coexpress ESK2 AbTCR and anti-CD33 costimulatory receptor (CSR). The CSR is composed of an anti-CD33 scFv (M195) linked to a partial CD28 molecule (consisting of the hinge, transmembrane, and intracellular domains of CD28) and included a Myc tag. The DNA sequences encoding the ESK2 AbTCR-CSR constructs (ESK2 AbTCR + anti-CD33 CSR) were cloned into a pCDH lentiviral vector (Systems Biosciences) for delivery into primary T cells (details in supplemental Materials).
Other methods (flow cytometry, animal experiments, and protein engineering) are described in detail in supplemental Materials.
Results
TCRm ESK2 is selective for WT1 RMF
The first TCRm to the WT1 RMF/HLA-A2 complex (known as ESK1) bound to the peptide/major histocompatibility complex such that the complementarity determining region 3 loops were focused over the α2 helix of the HLA, allowing for promiscuity to many other potential epitopes.18,19 Therefore, screening strategies for ESK2 development were designed to obtain TCRm mAbs that recognized more and centrally placed amino acid residues of the RMF peptide. In silico screening (Table 1) suggested that specificity to the central FPNA sequence of amino acids, and not the C- or N-terminal amino acids, should remove nearly all off-target 9-mer peptides identified in exosome screening with predicted affinity of <500 nM to HLA-A2 molecules. Selected phage clones no. 18 and no. 34 were converted to immunoglobulin G and bispecific T-cell engager (BiTE) formats for additional screening of binding to cell targets and cytotoxicity assays.
Homolog peptides identified in human exome by in silico screening
| Sequences tested for cross-reactivity to RMFPNAPYL∗ . | Epitope name . | Sequences found in exome (<1 μM Kd) . | Source protein . | HLA-A2 binding (KD prediction) . |
|---|---|---|---|---|
| X(LMV)FPNAPY(LVI) | WT1-RMF | RMFPNAPYL | WT1 | 7 nM |
| X(LMV)FPNAPY(LVI) And substitute any other position with any conserved amino acid | ABHD3-9-mer | SLYPSAPFL | Phospholipase ABHD3 | 7 nM |
| Same as previous row but with 10 residues instead | ABHD3-10-mer | SLYPSAPFLA | Phospholipase ABHD3 | 14 nM |
| AMFR | EMFPQVPYHL | Autocrine motility factor receptor | 148 nM | |
| Identified previously (Ref 20) | TSTP1-10-mer | RLFPNAKFLL | Tyrosyl protein sulfotransferase 1 | 27 nM |
| Sequences tested for cross-reactivity to RMFPNAPYL∗ . | Epitope name . | Sequences found in exome (<1 μM Kd) . | Source protein . | HLA-A2 binding (KD prediction) . |
|---|---|---|---|---|
| X(LMV)FPNAPY(LVI) | WT1-RMF | RMFPNAPYL | WT1 | 7 nM |
| X(LMV)FPNAPY(LVI) And substitute any other position with any conserved amino acid | ABHD3-9-mer | SLYPSAPFL | Phospholipase ABHD3 | 7 nM |
| Same as previous row but with 10 residues instead | ABHD3-10-mer | SLYPSAPFLA | Phospholipase ABHD3 | 14 nM |
| AMFR | EMFPQVPYHL | Autocrine motility factor receptor | 148 nM | |
| Identified previously (Ref 20) | TSTP1-10-mer | RLFPNAKFLL | Tyrosyl protein sulfotransferase 1 | 27 nM |
Potential off-target sequences were identified using ScanProsite (Expasy) to search the human exome (UniProtKB/Swiss-Prot) for homologous 9 amino acid peptides that had predicted affinity to HLA-A∗02:01 (HLA-A2) of <500 nM using NetMHCPan.
The rules used to identify potential off-target sequences are as follows: position no. 1 could be any amino acid “X”; position no. 2 and no. 9 could be any canonical anchor (LMV) and (LVI). Position no.3 to no.8 could be replaced with any conserved residues, shown hereafter, at their respective positions in RMFPNAPYL: F→Y = W; P→P; N→S = T = Q; A→V = I = G; Y→F = W. Additional homologous 10-mers were also included. TSTP1 was identified in a previous study.20
We tested the specificity of ESK2-clone no. 18 and no. 34 on T2 cells pulsed with RMF, mutant peptides, and exomic peptides that represent potential off-targets with high affinity of binding to HLA (Table 1). Both clones showed binding to T2 cells pulsed with RMF peptide.
Although ESK1 bound the WT1-AAA mutant peptide, and off-target peptide TSTP1, clone no. 18 did not bind to any of these peptides, and clone no. 34 only bound to the ABHD3-9mer peptide (Figure 1A). All the peptides showed HLA-A2 stabilization in T2 cells (Figure 1B). Differential binding profiles of ESK2 clones compared to ESK1 were demonstrated by alanine screening on T2 cells (Figure 1C; supplemental Table 1). ESK1 recognition largely depended on positions 1R and 4P of the RMF peptide,7,18,19 whereas clone no. 18 depended on positions 1R, 3F, 7P, and 8Y and clone no. 34 used residues 7P and 8Y. The A4P and A6G substitutions also showed reduced HLA-A2 stable expression, confounding interpretation of results at these positions (Figure 1D). Mutations on anchor residues in positions 2 and 9 would reduce the peptide binding and therefore these were left intact. These results demonstrated that binding of ESK1 was N-terminal dominated, clone no. 18 was broader and more centrally located, and clone no. 34 more C-terminal oriented. Clone no. 18 and no. 34 BiTEs had comparable affinities of 3 nM and 1.5 nM, respectively (supplemental Figure 1).
Binding of the ESK2 clone no. 18 and no. 34 and specificity of the epitope. (A) Binding of clones to T2 cells pulsed with or without indicated peptides. WT1 RMF or mutant peptides at a concentration of 50 μg/mL were pulsed onto T2 cells overnight. Cells were washed and stained with BiTEs of ESK2 clone no. 18 and no. 34 or ESK1 at 1 μg/mL. T2 cells alone or pulsed with irrelevant HLA-A2–binding peptide HPV-E7 (39) were used as controls. (B) In parallel, HLA-A2 expression was determined by staining the cells with the anti–HLA-A2 mAb BB7 clone. (C) WT1 RMF sequences were substituted with alanine at positions 1, 3, 4, 5, 7, and 8, or with glycine at position 6 indicated as WT1-A1 to WT1-A8 or WT1-G6, respectively (supplemental Table 1) and the binding of (C) ESK2-clone no. 18 and no. 34 and (D) HLA-A2 was determined by flow cytometric analysis. The data are representative results from 6 similar experiments. HPV, human papilloma virus.
Binding of the ESK2 clone no. 18 and no. 34 and specificity of the epitope. (A) Binding of clones to T2 cells pulsed with or without indicated peptides. WT1 RMF or mutant peptides at a concentration of 50 μg/mL were pulsed onto T2 cells overnight. Cells were washed and stained with BiTEs of ESK2 clone no. 18 and no. 34 or ESK1 at 1 μg/mL. T2 cells alone or pulsed with irrelevant HLA-A2–binding peptide HPV-E7 (39) were used as controls. (B) In parallel, HLA-A2 expression was determined by staining the cells with the anti–HLA-A2 mAb BB7 clone. (C) WT1 RMF sequences were substituted with alanine at positions 1, 3, 4, 5, 7, and 8, or with glycine at position 6 indicated as WT1-A1 to WT1-A8 or WT1-G6, respectively (supplemental Table 1) and the binding of (C) ESK2-clone no. 18 and no. 34 and (D) HLA-A2 was determined by flow cytometric analysis. The data are representative results from 6 similar experiments. HPV, human papilloma virus.
The specificity and cytotoxicity of ESK2-BiTEs against a panel of tumor cell lines was both WT1 and HLA-A2 dependent. Although clone no. 34 could bind to and kill various leukemia and solid tumor cells (Figure 2A-E), no binding or killing was observed with either the HLA-A2−/WT1+ cell line HL-60 or the HLA-A2+/WT1− cell line SKLY-16 (Figure 2F; Table 2). Unexpectedly, ESK2–no. 18 BiTE did not show cytotoxicity against any of the same positive tumor cells. The specific cytolytic activity of clone no. 34 was further confirmed in a 5-hour 51Cr release assay (Figure 2D-F). Overnight cultures showed even greater cytolytic activity. ESK1 BiTE showed slightly stronger cytotoxicity against the same target cells than ESK2–no. 34 BiTE, which could be because of a higher affinity of ESK1 than ESK2.
Specific recognition and killing of tumor cells by clone no. 34 BiTE. Recognition and cytolytic activity of the naturally presented WT1 RMF/A2 complex on the tumor cell surface by the ESK2 clone no. 18 and no. 34 BiTEs were probed. (A) MAC-1 T-cell lymphoma, (B) JMN mesothelioma, or (C) SW-620 colon cancer cell lines were incubated with PBMCs at an effector-to-target (E:T) ratio of 20:1, in the presence or absence of BiTEs and control BiTEs at the indicated concentrations overnight, and the cytotoxicity was measured by BLI. (D) BV173 CLL, (E) SET-2 AML, (F) or HL-60 AML cell lines were incubated with PBMCs at an E:T ratio of 20:1, in the presence or absence of clone no. 34 BiTE or control at the concentrations of 1 μg/mL, 0.3 μg/mL, or 0.1 μg/mL for 5 hours and the cytotoxicity was measured using a 51Cr-release assay. The mean shown is the average of triplicate microwells ± standard deviation. The data are representative of 10 experiments. The effector cells were used from several different donors; whereas differences among the experimental groups were similar, the baselines were variable among the individuals; therefore, only representative data are shown.
Specific recognition and killing of tumor cells by clone no. 34 BiTE. Recognition and cytolytic activity of the naturally presented WT1 RMF/A2 complex on the tumor cell surface by the ESK2 clone no. 18 and no. 34 BiTEs were probed. (A) MAC-1 T-cell lymphoma, (B) JMN mesothelioma, or (C) SW-620 colon cancer cell lines were incubated with PBMCs at an effector-to-target (E:T) ratio of 20:1, in the presence or absence of BiTEs and control BiTEs at the indicated concentrations overnight, and the cytotoxicity was measured by BLI. (D) BV173 CLL, (E) SET-2 AML, (F) or HL-60 AML cell lines were incubated with PBMCs at an E:T ratio of 20:1, in the presence or absence of clone no. 34 BiTE or control at the concentrations of 1 μg/mL, 0.3 μg/mL, or 0.1 μg/mL for 5 hours and the cytotoxicity was measured using a 51Cr-release assay. The mean shown is the average of triplicate microwells ± standard deviation. The data are representative of 10 experiments. The effector cells were used from several different donors; whereas differences among the experimental groups were similar, the baselines were variable among the individuals; therefore, only representative data are shown.
Tumor cell lines used for ESK2 clone no. 34 binding and cytotoxicity
| Tumor cell name . | Tumor tissue type . | RMF/HLA-A2 complex expression . | HLA-A2 expression . | Cytotoxicity (using BiTE) . |
|---|---|---|---|---|
| AML-14 | AML | + | +++ | +++ |
| BV173 | Chronic myeloid leukemia (B-blastic) | + | ++ | ++ |
| SET-2 | AML | + | ++ | ++ |
| OCI-AML-2 | AML | + | ++ | NT |
| JMN | Mesothelioma | + | ++ | ++ |
| MAC-1 | T-cell lymphoma | ++ | +++ | +++ |
| MAC-2A | T-cell lymphoma | ++ | +++ | +++ |
| OV56 | Ovarian cancer | + | ++ | ++ |
| SW-620 | Colon cancer | + | ++ | ++ |
| HL-60 | AML | − | − | − |
| SKLY-16 | NHL | − | + | − |
| Tumor cell name . | Tumor tissue type . | RMF/HLA-A2 complex expression . | HLA-A2 expression . | Cytotoxicity (using BiTE) . |
|---|---|---|---|---|
| AML-14 | AML | + | +++ | +++ |
| BV173 | Chronic myeloid leukemia (B-blastic) | + | ++ | ++ |
| SET-2 | AML | + | ++ | ++ |
| OCI-AML-2 | AML | + | ++ | NT |
| JMN | Mesothelioma | + | ++ | ++ |
| MAC-1 | T-cell lymphoma | ++ | +++ | +++ |
| MAC-2A | T-cell lymphoma | ++ | +++ | +++ |
| OV56 | Ovarian cancer | + | ++ | ++ |
| SW-620 | Colon cancer | + | ++ | ++ |
| HL-60 | AML | − | − | − |
| SKLY-16 | NHL | − | + | − |
The presentation of WT1 RMF/HLA-A2 complex is measured by binding to tumor cells using mouse ESK2 no. 34 (3 μg/mL). Binding of the ESK2 was ranked as fold increase over isotype control: +, 1.5-fold to threefold increases; ++, >fivefold to 10-fold increases; and −, no binding at all.
HLA-A2 expression was measured by mouse anti-HLA-A2 mAb, clone BB7; +++, >80-fold increases over isotype control; ++, 40- to 80-fold increases; and +, twofold to 10-fold increases.
Cytotoxicity was tested by ESK2 no. 34 BiTE at various concentrations (examples shown in Figure 2). Cytotoxicity mediated by ESK2 no. 34 BiTE was measured by either standard 51Cr release (5 hours) or luciferase assay (overnight). The killing activity depends on the dosage and the incubation duration as shown in Figure 2. ++, threefold to fivefold increases in the killing over isotype control; +++, >10-fold killing.
WT1 expression of the cell lines used herein were tested for their WT messenger RNA or protein by us and others in previous studies.9,10,13,16 The data are summarized from 3 to 7 experiments, depending on cell lines.
CML, chronic myeloid leukemia; NHL, non-Hodgkin lymphoma; NT, not tested.
Importantly, neither significant binding nor cytotoxicity of clone no. 34 was seen against whole blood including CD15+ neutrophils or normal CD3 T cells and against CD33+ monocytes of peripheral blood mononuclear cells (PBMCs) from healthy HLA-A2–positive or –negative donors (Table 3; supplemental Figure 2A-E). There was minimal binding to CD19+ B cells (supplemental Figure 2F-G). However, clone no. 34 BiTE demonstrated no cytotoxicity against T cells, B cells, or monocytes/macrophages in PBMCs or neutrophils in whole blood from healthy HLA-A2+ donors. Furthermore, clone no. 34 showed no binding or killing against normal adrenal microvascular endothelial tissue, renal glomerular endothelial tissue, cardiac myocytes, and testicular endothelial cells (Table 3).
Summary of binding and cytotoxicity of ESK2 no. 34 on a panel of normal hematopoietic cells and nonhematopoietic cells
| Hematopoietic cells from healthy donors . | |||||
|---|---|---|---|---|---|
| Whole blood (binding/cytotoxicity) . | PBMCs (binding/cytotoxicity) . | ||||
| . | HLA-A2+ . | HLA-A2− . | . | HLA-A2+ . | HLA-A2− . |
| Neutrophils (CD15+) | Neg/neg | Neg/neg | T cells (CD3+) | Neg/neg | Neg/neg |
| Monocytes (CD33+) | Neg/neg | Neg/neg | Monocytes (CD33+) | Neg/neg | Neg/neg |
| Lymphocytes (CD45+) | 4%+/neg | Neg/neg | Monocytes (CD14+) | Neg/neg | Neg/neg |
| B cells (CD19+) | 2%-3%/neg | Neg/neg | |||
| Hematopoietic cells from healthy donors . | |||||
|---|---|---|---|---|---|
| Whole blood (binding/cytotoxicity) . | PBMCs (binding/cytotoxicity) . | ||||
| . | HLA-A2+ . | HLA-A2− . | . | HLA-A2+ . | HLA-A2− . |
| Neutrophils (CD15+) | Neg/neg | Neg/neg | T cells (CD3+) | Neg/neg | Neg/neg |
| Monocytes (CD33+) | Neg/neg | Neg/neg | Monocytes (CD33+) | Neg/neg | Neg/neg |
| Lymphocytes (CD45+) | 4%+/neg | Neg/neg | Monocytes (CD14+) | Neg/neg | Neg/neg |
| B cells (CD19+) | 2%-3%/neg | Neg/neg | |||
| Nonhematopoietic normal cells . | ||||
|---|---|---|---|---|
| . | WT1 mRNA expression . | HLA-A2 expression . | Binding . | Cytotoxicity . |
| Adrenal microvascular endothelial cells | +++ | Neg | Neg | Neg |
| Renal glomerular endothelial cells | +++ | Pos | Neg | Neg |
| Cardiac myocytes | NT | Neg | Neg | Neg |
| Testicular endothelial cells | NT | Pos | Neg | Neg |
| Nonhematopoietic normal cells . | ||||
|---|---|---|---|---|
| . | WT1 mRNA expression . | HLA-A2 expression . | Binding . | Cytotoxicity . |
| Adrenal microvascular endothelial cells | +++ | Neg | Neg | Neg |
| Renal glomerular endothelial cells | +++ | Pos | Neg | Neg |
| Cardiac myocytes | NT | Neg | Neg | Neg |
| Testicular endothelial cells | NT | Pos | Neg | Neg |
WT1 messenger RNA (mRNA) expression was measured by reverse transcription polymerase chain reaction, as previously described.16 Binding to normal cells was determined by ESK2 no. 34 mouse immunoglobulin G1 (3 μg/mL) over control isotype staining. Cytotoxicity was measured by ESK2 no. 34 BiTE at concentrations of 0.1, 1, or 10 μg/mL in standard 51Cr-release assay as described in “Materials and methods.”
For hematopoietic cells, the data are representative of 6 donors.
“+++” indicates 104 to 105 increases of WT1 transcript over negative control cells.
NT, not tested; Pos, positive (≥20-fold increase over isotype control staining for HLA-A2; BB7.2 clone); Neg, negative (no increase over isotype control staining).
The 14 ESK2 clones were also tested in the AbTCR format as candidates for T-cell therapy against AML. The Fab of ESK2 antibodies were fused with the C-terminal of γδTCR and constructed into lentivirus for T-cell transduction. The AbTCR clones in primary T cells were tested for cytotoxicity and specificity by coculture with T2 cells loaded with RMF or other control peptides (supplemental Figure 3A). Eight different cytolytic AbTCRs demonstrated cytotoxicity against RMF-loaded T2 cells and low cross-reactivity with other peptides. Clone no. 2, no. 15, no. 18, and no. 34 stood out for significantly higher killing activity than other clones after 16-hour coculture with SK-HEP1-WT1 minigene, ectopically expressing the RMF peptide region as a minigene (supplemental Figure 3B). Of these clones, AbTCR T cells of clones no. 18 and no. 34 showed specific killing activity, especially against the SKM-1 AML line and not K562 (WT1+/ HLA-A∗02:01−). These 2 clones were selected as the final leading ESK2 AbTCR T-cell clones (supplemental Figure 3C).
Noticeably, ESK2 AbTCR T-cell potency was significantly higher against SKM-1 than against SET-2. Because both the “first signal” (provided by AbTCR) and costimulatory signaling are essential for full activation of T cells, we investigated the expression of CD28/4-1BB ligands expressed on these 2 AML cell lines (supplemental Figure 3D). Flow cytometry detected high CD86 (a CD28 ligand) and CD137L (a 4-1BB ligand) levels on SKM-1, whereas only minor CD80 (a CD28 ligand) expression was detected on SET-2. We hypothesized that the abundant costimulation signal from SKM-1 contributed to its killing by ESK2 AbTCR T cells. To test this hypothesis, we added anti-human CD28 antibody into the cocultures to activate CD28 signal pathway in AbTCR T cells regardless of CD28 ligand levels on tumor cells. A significant boost of killing activity against SET-2 was observed in contrast to against SKM-1 (supplemental Figure 3E), emphasizing the importance of costimulatory signaling for robust AbTCR T-cell activation. This hypothesis was further validated by the interferon γ secretion from ESK2 AbTCR T cells with or without anti-CD33 CSR after coculture with SET-2 (supplemental Figure 4).
Cytotoxicity of ESK2 AbTCR-CSR against myeloid leukemia requires AbTCR signaling and is augmented by costimulatory signals
To provide costimulatory signals to ESK2 T cells, we coupled clone no. 18 and no. 34 AbTCR with the scFv of anti-CD33 lintuzumab22 that was fused to the transmembrane and intracellular signaling domains of CD28. We hypothesized that the T cells expressing such a dual-receptor combination will be fully activated with both TCR/CD3 and CD28 costimulatory signals in a way that resembles the 2-signal and gate model of natural T-cell activation, hence increasing the specificity against CD33+/WT1+ tumor cells. Meanwhile, with the 2-component activation design, on-target/off-tumor and off-target toxicities should be reduced. A scheme of the AbTCR-CSR and its constructs is shown in Figure 3A, and the vector map in supplemental Figure 5A. Transduction efficiency of the AbTCR-CSR clones no. 18 and no. 34 and their cell expansion are shown in supplemental Figure 5B-E.
Cytotoxicity of AbTCR or AbTCR-CSR against AML cells. (A) A cartoon depicting the design of the AbTCR and the costimulatory receptor CSR only, which, together, form the AbTCR-CSR. AbTCR is the primary signal, CSR is the secondary signal. The combination is known as ARTEMIS 2.0. ESK2 AbTCR or ESK2 AbTCR-CSR T cells (B-C) were incubated with the indicated leukemia target cells at an E:T ratio of 1:1, overnight. The cytotoxicity was measured by luciferase-based assay. Each data point was the average of triplicate cultures ± standard deviation, and representative of 3 similar experiments with different donors. To test whether the cytotoxicity of AbTCR-CSR T cells required the primary signal, (D) AML-14, (E) HL-60, or (F) SKOV3/A2 cell lines were labeled with CFSE, washed, and incubated with mock T cells, CSR cells (ESK2 is replaced with an irrelevant Fab AbTCR as the recognition receptor), ESK2 AbTCR-CSR T cells no. 18, or ESK2 AbTCR-CSR T cells no. 34 at an E:T ratio of 1:1, overnight. The cells were harvested, washed, and stained with mAb to CD33 and subjected to flow cytometry. The analysis was performed by gating on larger tumor cells based on forward and side scatters, and the percentage of CD33+ cells was shown in the CFSE+ target population.
Cytotoxicity of AbTCR or AbTCR-CSR against AML cells. (A) A cartoon depicting the design of the AbTCR and the costimulatory receptor CSR only, which, together, form the AbTCR-CSR. AbTCR is the primary signal, CSR is the secondary signal. The combination is known as ARTEMIS 2.0. ESK2 AbTCR or ESK2 AbTCR-CSR T cells (B-C) were incubated with the indicated leukemia target cells at an E:T ratio of 1:1, overnight. The cytotoxicity was measured by luciferase-based assay. Each data point was the average of triplicate cultures ± standard deviation, and representative of 3 similar experiments with different donors. To test whether the cytotoxicity of AbTCR-CSR T cells required the primary signal, (D) AML-14, (E) HL-60, or (F) SKOV3/A2 cell lines were labeled with CFSE, washed, and incubated with mock T cells, CSR cells (ESK2 is replaced with an irrelevant Fab AbTCR as the recognition receptor), ESK2 AbTCR-CSR T cells no. 18, or ESK2 AbTCR-CSR T cells no. 34 at an E:T ratio of 1:1, overnight. The cells were harvested, washed, and stained with mAb to CD33 and subjected to flow cytometry. The analysis was performed by gating on larger tumor cells based on forward and side scatters, and the percentage of CD33+ cells was shown in the CFSE+ target population.
We tested the cytotoxicity of both ESK2-18 and ESK2-34 AbTCR and AbTCR-CSR against a panel of leukemia cell lines. AbTCR of both clones were able to kill the AML cell lines that were triple positive for WT1/HLA-A2 and CD33. The cytotoxicity was further enhanced, depending on the target cell line, in AbTCR-CSR T cells (Figure 3A). However, no significant enhancement of the cytotoxicity by AbTCR-CSR T cells was observed against the cell lines that were CD33− such as BV173, MAC-2A, and SKLY-16. No cytotoxicity against HL-60, which is WT1+ and CD33+ but HLA-A2−, showing that the primary signal targeting WT1 RMF is a critical factor for cytotoxicity (Figure 3B). Phenotypes of the cell lines are listed in supplemental Table 2. These results demonstrated our hypothesis that ESK2-CD33 AbTCR-CSR T cells were potent and specific cells against target cells simultaneously expressing all 3 target proteins.
Next, we further assessed the coordination and necessity of the 2 stimulatory signals. We engineered AbTCR-CSR T cells in which ESK2 Fab was replaced with an irrelevant Fab, leaving the CD33 costimulatory receptor as before, termed as CSR only. Four target cell lines were tested: AML-14 (WT1+/CD33+/HLA−A2+); HL-60 and K562 (WT1+/CD33+/HLA−A2−); and SKOV3/A2 (WT1+/CD33−/HLA−A2+ transduced ovarian cancer cell line) (supplemental Table 2). Carboxyfluorescein succinimidyl ester (CFSE)-labeled target-cell killing was measured by flow cytometry analysis after overnight incubation with the effectors and stained with anti-CD33 mAb. Target cells were gated as larger tumor cells based on forward and side scatters, to exclude the smaller effector T cells, and we measured the percentage of CD33 and CFSE double-positive target cells (Figure 3C-D). With the control mock T-cell effectors, there were 88% CD33+/CFSE+ AML-14 target cells remaining. AbTCR-CSR T cells with the CSR showed downmodulation of detectable cell surface CD33 levels on target cells (because of the presence of the CD33 scFv on the effectors that masked or downregulated surface CD33); however, the percentage of live target cells was similar to the control mock T cells at 93% (right quadrants). This showed that the first signal is required to activate T cells to kill. AbTCR-CSR T cells of both clone no. 18 and clone no. 34 not only showed downmodulation of CD33 levels on target cells but also killing (50%-55%; Figure 3C, right quadrants). In HL-60 cells (HLA−A2−), no killing was seen with any effector cells, although CD33 surface levels were also reduced in the CSR-only group (Figure 3D), thus again showing the need for the first signal. In another experiment, K562 cells (HLA-A2−) showed similar results to that of HL-60. These data support a superior specificity profile of ESK2 AbTCR-CSR T cells. No binding of CD33 nor killing of SKVO3/A2 ovarian cancer cells was observed, because the cells do not express CD33, and percentages of all CFSE+ cells remain similar in all 4 groups, showing the requirement for the second CSR (Figure 3E).
ESK2 AbTCR or AbTCR-CSR T cells were then tested for their ability to kill primary AML samples. Four CD33+ AML primary samples were tested as targets. The elimination of CD33+ populations were calculated in comparison with the mock T-cell groups (Table 4.) There was a variable degree of killing among the samples by the effector AbTCR-CSR T cells, which could be due to the heterogeneity of the CD33 on primary samples (Table 4). In general, clone no. 18 showed more potent killing than clone no. 34 in both AbTCR and AbTCR-CSR formats, and CSR-only T cells did not show the reduction of CD33+ cells. No significant killing was detected against HLA-A∗02:01–negative sample no. 120B. Representative flow plots from sample no. 92C are shown in supplemental Figure 6. These results demonstrated that the ESK2 AbTCR and AbTCR-CSR T cells were cytolytic against the primary AML cells that were WT1+/CD33+/HLA-A2+.
Reduction of primary AML by AbTCR and AbTCR-CSR T cells
| Primary AML . | Reduction of CD33+ cells (%) . | HLA-A∗02:01 . | WT1 RMF/HLA-A2 complex expression . | CD33 expression . | ||||
|---|---|---|---|---|---|---|---|---|
| CSR only . | AbTCR no. 18 only . | AbTCR no. 34 only . | AbTCR-CSR no. 18 . | AbTCR-CSR no. 34 . | ||||
| 60D | None | 73 | 28 | 90 | 16 | + | + | 99% |
| 114B | None | 35 | 38 | 81 | 17 | + | + | 54% |
| 92C | None | 57 | 69 | 69 | 74 | + | + | 77% |
| 120B | None | 5.6 | None | None | 2.3 | − | − | 40% |
| AML-14 | None | 41 | 55 | 74 | 55 | + | + | 99% |
| Primary AML . | Reduction of CD33+ cells (%) . | HLA-A∗02:01 . | WT1 RMF/HLA-A2 complex expression . | CD33 expression . | ||||
|---|---|---|---|---|---|---|---|---|
| CSR only . | AbTCR no. 18 only . | AbTCR no. 34 only . | AbTCR-CSR no. 18 . | AbTCR-CSR no. 34 . | ||||
| 60D | None | 73 | 28 | 90 | 16 | + | + | 99% |
| 114B | None | 35 | 38 | 81 | 17 | + | + | 54% |
| 92C | None | 57 | 69 | 69 | 74 | + | + | 77% |
| 120B | None | 5.6 | None | None | 2.3 | − | − | 40% |
| AML-14 | None | 41 | 55 | 74 | 55 | + | + | 99% |
Primary AML samples were labeled with either CFSE or far-red and coincubated with ESK2 AbTCR or AbTCR-CSR T cells at an effector-to-target ratio of 1:1, overnight. The cells were harvested and were stained with anti-CD33 mAb and analyzed by flow cytometry. The cells were gated on live cells and the same gates were applied to all groups. The percentage of CD33 and CFSE-positive (or far-red–positive) cells in the live cell gates were analyzed (as shown in supplemental Figure 6), and the percentage of total CD33+CFSE/far-red+ cells were calculated (including CD33low cells in AbTCR-CSR groups).
Percentage reduction of the AML cells by AbTCR, AbTCR-CSR T cells was calculated by comparison with mock T cells. HLA-A2, and CD33 expression were measured by flow cytometric analysis.
WT1 expression was assessed by ESK1 and ESK2 staining. AML-14 is a positive control AML cell line.
+, ≥1.5-fold increase over the isotype control staining for WT1 RMF/HLA-A2; +, ≥20 fold over isotype for HLA-A2 and CD33; and none, 0% to 1.5% reduction over mock T-cell groups.
In addition, we asked whether such a dual targeting construct could have not only cis-activation from both signals on the same cell but also trans-activation from a cell expressing the primary target and killing of cells that expressed the secondary target. AbTCR-CSR no. 18 and no. 34 T cells were incubated with JMN (WT1+/HLA−A2+/CD33−) or MSTO (WT1+/HLA−A2−/CD33−) cells overnight and then harvested. The effector cells were then coincubated with the HL-60 cell line (only CD33+) to test whether the AbTCR-CSR T cells could acquire activation signal from the primary signaling domain, resulting in killing against the target cells expressing only the CSR (CD33). AbTCR-CSR T cells sensitized with JMN were able to kill AML-14 (triple positive) but not HL-60 (primary signal negative; supplemental Figure 7.) This suggests that under these transfer conditions and with these lines, cissignaling is required. However, transsignaling may still occur in settings in vivo in which multiple target cells are in proximity for long periods of time.
AbTCR-CSR T cells were not cytotoxic to normal hematopoietic cells
Although ESK2 clone no. 34 was not cytotoxic in immunoglobulin G or BiTE format against normal hematopoietic cells, a cellular format might substantially increase the avidity or potency, thereby resulting in killing with a single signal. PBMCs from either HLA-A2–positive or –negative donors were CFSE labeled and incubated with AbTCR-CSR effector cells overnight. The cells were then stained with mAbs to T-cell, B-cell, and monocyte markers to determine the depletion of subpopulations. For monocytes, cells were stained with anti-CD33 mAb and zombie dye to determine the percentage death in the CD33+/CFSE+ target population. Mock T cells and CSR-only T cells showed little killing of the positive control cell line AML-14, whereas AbTCR-CSR clone no. 18 and, to a lesser extent, no. 34 were cytolytic (Figure 4A). In contrast, no killing was observed in CD33+ cells compared with in control mock T cells among the 4 groups in both HLA-A∗02:01–positive (Figure 4B) or –negative donors (Figure 4C). These data are summarized in Figure 4D. No significant reduction was observed in CD3+ T-cell and CD19+ B-cell compartments in both HLA-A∗02:01–positive or –negative donors treated with all 4 groups of effectors (Figure 4E). The results were confirmed in a second set of donors with a similar detailed analysis. In addition, in earlier studies, no. 34 AbTCR-CSR cells showed no killing against PBMCs from multiple healthy donors in lactate dehydrogenase.
No cytotoxicity of AbTCR-CSR was observed against normal PBMCs. Mock T cells, CSR (with irrelevant Fab AbTCR), ESK2 AbTCR-CSR clone no. 18, or AbTCR-CSR clone no. 34 were incubated with (A) CFSE-labeled AML-14 (as a positive control), (B) PBMCs from HLA-A2 positive donor, or (C) negative donor at an E:T ratio of 1:1, overnight. The cells were harvested, washed, and stained with mAb to CD33 and zombie dye (to stain dead cells) and analyzed by flow cytometry. (D) The flow plots show a representative profile of CD33 vs CFSE double-positive cells and the percentage of CD33+ cell death was summarized. (E) In the same experiments, CFSE+ target PBMCs were also stained with CD3 and CD19 and the percentage of each lineage cells are shown in bar graphs. The data represent 1 of 4 separate experiments with different donors.
No cytotoxicity of AbTCR-CSR was observed against normal PBMCs. Mock T cells, CSR (with irrelevant Fab AbTCR), ESK2 AbTCR-CSR clone no. 18, or AbTCR-CSR clone no. 34 were incubated with (A) CFSE-labeled AML-14 (as a positive control), (B) PBMCs from HLA-A2 positive donor, or (C) negative donor at an E:T ratio of 1:1, overnight. The cells were harvested, washed, and stained with mAb to CD33 and zombie dye (to stain dead cells) and analyzed by flow cytometry. (D) The flow plots show a representative profile of CD33 vs CFSE double-positive cells and the percentage of CD33+ cell death was summarized. (E) In the same experiments, CFSE+ target PBMCs were also stained with CD3 and CD19 and the percentage of each lineage cells are shown in bar graphs. The data represent 1 of 4 separate experiments with different donors.
Next, we tested whether the AbTCR-CSR T cells had any cytotoxicity against neutrophils (heterogeneously CD33+). Neutrophils isolated from HLA-A∗02:01–positive or –negative donors (Figure 5A-B) were incubated with AbTCR-CSR T cells and stained with CD15 for neutrophils and CD3 for the effector T cells. There was no reduction in the percentage of CD15+ neutrophils in either HLA-A∗02:01–positive or –negative donors (Figure 5C). However, AML-14 was killed by both AbTCR-CSR clones no. 18 and no. 34 in the same experiment as a control (Figure 5D).
No cytotoxicity of AbTCR-CSR against neutrophils from normal donors. (A) Neutrophils from HLA-A∗02:01–positive donor or (B) HLA-A∗02:01–negative donor were isolated using a human whole-blood neutrophil isolation kit (Miltenyi) and incubated with mock T cells, CSR only, or AbTCR-CSR clone no. 18 or no. 34 at an E: T ratio of 1:1, overnight. The cells were stained with CD15 (for neutrophils) or CD3 (AbTCR-CSR T cells). The percentage of CD15+CFSE+ cells after coculture are shown in panel C and the positive control cell line AML-14 is shown in panel D. Because AML-14 cells do not express CD15, CD33 was used for its marker. The data represents 1 set of results from 4 independent donors.
No cytotoxicity of AbTCR-CSR against neutrophils from normal donors. (A) Neutrophils from HLA-A∗02:01–positive donor or (B) HLA-A∗02:01–negative donor were isolated using a human whole-blood neutrophil isolation kit (Miltenyi) and incubated with mock T cells, CSR only, or AbTCR-CSR clone no. 18 or no. 34 at an E: T ratio of 1:1, overnight. The cells were stained with CD15 (for neutrophils) or CD3 (AbTCR-CSR T cells). The percentage of CD15+CFSE+ cells after coculture are shown in panel C and the positive control cell line AML-14 is shown in panel D. Because AML-14 cells do not express CD15, CD33 was used for its marker. The data represents 1 set of results from 4 independent donors.
An important question remained as to whether normal hematopoietic human progenitor cells, which are reportedly CD33+,22,23 would be spared by ESK2 AbTCR-CSR. Therefore, we assessed colony-forming unit assays from cord blood isolated HLA-A02+/CD34+ hematopoietic stem cells. There was no reduction in the ability to form colonies among the stem cells (supplemental Figure 8A-B). Interestingly, this sparing of progenitor cells also could be explained by the low expression of HLA molecules on the CD34+ cell surfaces, thus protecting them from killing (supplemental Figure 8C-D). Similar results were observed in a total of 5 cord blood samples.
ESK2 AbTCR-CSR T-cell therapy of human AML cells in NSG mice
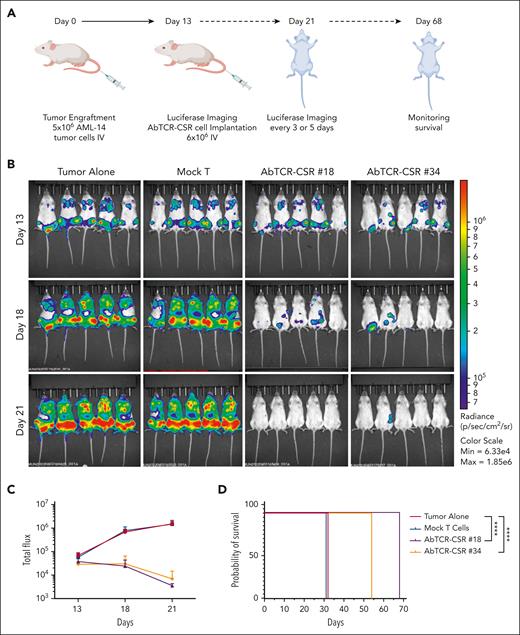
We tested the efficacy of the ESK2 AbTCR-CSR cells in 2 models in vivo. First, NSG mice received xenografts IV with AML-14 cells 13 days before treatment (Figure 6A), yielding disseminated leukemia in the lung, spleen, and bone marrow (Figure 6B). AbTCR-CSR clone no. 18 and no. 34 cells administered by IV injection resulted in a reduction of tumor sizes within 5 days and improved through 8 days. Both AbTCR-CSR clones no. 18 and clone no. 34 nearly eliminated leukemia in all mice with 100-fold bioluminescence (BLI) reductions in comparison with control groups on day 21(Figure 6C). The reduction of tumor cells correlated with the significant prolongation in survival in the AbTCR-CSR clone no. 18– and clone no. 34–injected groups (Figure 6D).
AbTCR-CSR therapeutic trial in an AML animal model. (A) Human AML cell line AML-14 cells (5 million) were injected IV into NSG mice and leukemia engraftment was confirmed on day 13 by bioluminescent imaging. (B) AbTCR-CSR clone no. 18, AbTCR-CSR clone no. 34, or control mock T cells (6 million cell per mouse) were injected IV, and tumor burden was monitored by BLI on days 18 and 21. (C) Mean tumor burden was calculated by summing the luminescence signal of each mouse and presenting the average signal for each group (n = 5 per group). (D) Survival of mice from experimental groups; a comparison of the differences of clone no. 18 or no. 34 vs the 2 control groups was performed using the Mantel-Cox test; ∗∗∗∗P < .0001.
AbTCR-CSR therapeutic trial in an AML animal model. (A) Human AML cell line AML-14 cells (5 million) were injected IV into NSG mice and leukemia engraftment was confirmed on day 13 by bioluminescent imaging. (B) AbTCR-CSR clone no. 18, AbTCR-CSR clone no. 34, or control mock T cells (6 million cell per mouse) were injected IV, and tumor burden was monitored by BLI on days 18 and 21. (C) Mean tumor burden was calculated by summing the luminescence signal of each mouse and presenting the average signal for each group (n = 5 per group). (D) Survival of mice from experimental groups; a comparison of the differences of clone no. 18 or no. 34 vs the 2 control groups was performed using the Mantel-Cox test; ∗∗∗∗P < .0001.
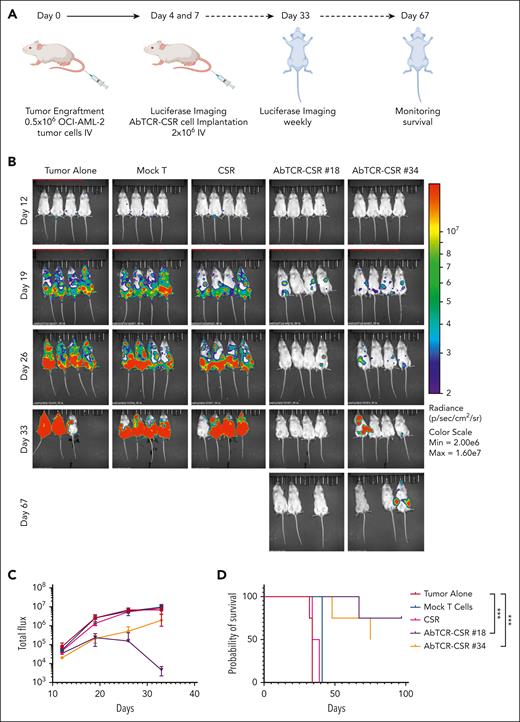
In the second, more aggressive AML model, effector cells were administered by IV injections on days 4 and 12 after OCI-AML-2 cells were xenografted (Figure 7A). Two doses of cells were used to treat this more rapidly growing cell line. Substantial tumor inhibition was seen in the groups of mice that received ESK2 AbTCR-CSR T cells. Mice that received clone no. 18 and no. 34 were largely tumor free by BLI analysis (Figure 7B). One tumor-positive mouse died on day 48. On day 67 after tumor injection, AbTCR-CSR–treated groups still were largely tumor free, except for 1 mouse that died tumor free without explanation; the remaining 3 mice were euthanized on day 75, as a termination of the experiment. Two tumor-free mice were euthanized prematurely, although tumor free, as a result of an early termination error by IACUC veterinarians at the mouse facility. Total BLI flux and survival curves showed strong inhibition of tumor growth by both clones no. 18 and no. 34 AbTCR-CSR T cells (Figure 7C-D). Importantly, mice treated with AbTCR-CSR T cells with irrelevant primary recognition domain and the CD33 reactive costimulatory domain (the CSR-only group) did not show any tumor inhibition, which was consistent with in vitro data, demonstrating the indispensability of the primary signal for any cytotoxicity from AbTCR-CSR T cells in vivo. Although there are multiple potential causes of tumor relapse in these xenograft models, short persistence of the human effector cells in mice and insufficient dosing regimens are more likely causes of relapse than antigen escape. Optimization of scheduling for long-term survival will await future human applications.
AbTCR-CSR therapeutic trial in an OCI-AML-2 human AML animal model. OCI-AML-2 cells (0.5 million) were injected IV into NSG mice. Groups were blindly assigned to treatment groups. (A) Mock T cells, CSR-only cells, or ESK2 AbTCR-CSR cells (1 million) were injected IV on day 4 and day 12 after tumor cell injection. (B) Tumor burden was assessed by BLI on the indicated days. (C) Mean tumor burden was calculated by summing the luminescent signal of each mouse, and the average signal for each group (n = 4 per group) was plotted. (D) Survival of mice from experimental groups. A comparison of the differences of clone no. 18 or no. 34 vs the 2 control groups was calculated using the Mantel-Cox test; ∗∗∗P < .001. Max, maximum; min, minimum.
AbTCR-CSR therapeutic trial in an OCI-AML-2 human AML animal model. OCI-AML-2 cells (0.5 million) were injected IV into NSG mice. Groups were blindly assigned to treatment groups. (A) Mock T cells, CSR-only cells, or ESK2 AbTCR-CSR cells (1 million) were injected IV on day 4 and day 12 after tumor cell injection. (B) Tumor burden was assessed by BLI on the indicated days. (C) Mean tumor burden was calculated by summing the luminescent signal of each mouse, and the average signal for each group (n = 4 per group) was plotted. (D) Survival of mice from experimental groups. A comparison of the differences of clone no. 18 or no. 34 vs the 2 control groups was calculated using the Mantel-Cox test; ∗∗∗P < .001. Max, maximum; min, minimum.
Discussion
The well-characterized cell surface targets for AML, such as CD33 and CD123, are expressed both on myeloid leukemia blasts and on healthy hematopoietic stem cells and progenitor cells.4,24 In case of AML, prolonged myeloid depletion after CD33-specific treatments are life threatening.25 To avoid such clinical outcomes from therapies depleting normal progenitors, we explored a cell therapy strategy that concomitantly targeted 2 antigens highly expressed on AML, WT1 and CD33. WT1 is a validated target that offers relative tumor specificity with a selective expression profile in AML cell lines and blasts but rarely on normal cells.11,15,25-27 However, because WT1 is intracellular, it can only be accessed with a TCR or TCRm, which are generally promiscuous, allowing for off-target toxicity.28
In a recent clinical trial, AbTCR-CSR T cells directed against CD19 on both the primary AbTCR and the costimulatory CSR were tested in patients with relapsed/refractory B-cell lymphoma.21 The study demonstrated that AbTCR-CSR T-cell therapy was well tolerated, with high response rates and no severe cytokine release syndrome. Furthermore, the T cells exhibit robust in vivo expansion and persistence.
Our hypothesis that dual targeting improved specificity was confirmed in vitro and in vivo: when cocultured with PBMC of healthy HLA-A2+ or HLA-A2− donors, ESK2 AbTCR-CSR did not kill cell lines that express only CD33 but are WT1− or HLA-A2− or kill normal CD33+ cells, such as monocytes and neutrophils. Moreover, T cells expressing the anti-CD33 CSR alone were not cytolytic in in vivo AML tumor models. The enhancing function of costimulatory signal in boosting ESK2 AbTCR T-cell activity was verified as the killing activity against a panel of leukemia cell lines and primary AML samples. Additionally, T-cell activation as measured by interferon-γ released from ESK2 AbTCR T cells was dramatically increased by the CD33 CSR. The use of γδTCR cells for primary signal that is directed to a cancer-selective intracellular target WT1 RMF/HLA and then targeting of cell surface CD33 for the second signal closely mimics the usual synapse-directed activation pathways and yields more typical T-cell activity. γδTCR usage avoids the problem of receptor misparing with αβTCR and could also reduce GVHD risk.
Cross-reactivity with potential toxicity is a characteristic of both TCR and TCRm mAbs. Other peptides in the proteome may share amino acid homologies or physicochemical features that also allow binding.29,30 In the AbTCR-CSR format, both clones no. 34 and no. 18 showed no significant cytotoxicity toward normal hematopoietic cells. Furthermore, no cytotoxicity was observed in CD34+ hematopoietic stem cells from HLA-A2–positive or –negative donors. Interestingly, although it was reported that a low frequency of hematopoietic stem cells express WT1 messenger RNA, we found little expression of HLA-A2 on CD34+ cells; hence, WT1 epitope presentation may be less concerning in these cells. This observation may explain the safety of a previous clinical trial using WT1 RMF-TCR transgenic T cells.15
We hypothesized that WT1-selective AbTCR complemented by a CD33-reactive CSR in a single cell would allow specificity, as well as better T-cell activation and persistence. Therefore, the ESK2 AbTCR-CSR platform offered a solution for dual targeting of tumor antigens. Signaling provided by the CD33 CSR may also lower the antigen density threshold for the WT1-specific AbTCR,31 thus, achieving more specific potency against AML cells.
Improved target specificity of the AbTCR-CSR is also largely attributed to the initial extensive selection process of the ESK2 mAbs. Clone no. 34 demonstrated tumor-selective binding and cytotoxicity against AML, chronic myeloid leukemia, and other types of solid tumor cells in a WT1- and HLA-A2–restricted manner but not against normal hematopoietic cells (neutrophils, T cells, B cells, and monocytes) and nonhematopoietic cells from either HLA-A∗02:01–positive or –negative donors. In contrast, although clone no. 18 in a BiTE format was not cytotoxic, it was even more active than clone no. 34 in the AbTCR-CSR format. This discrepancy may be because of some conformational issues in the BiTE construction, which are not uncommon in mAb engineering processes; however, it is beyond the scope of this paper.32 Each format could have its advantages as a therapeutic agent for possible future clinical applications: whereas clone no. 18 can be used as an AbTCR-CSR, clone no. 34 can be used as a BiTE. The smaller molecular weight and easy penetration of BiTEs may be an ideal format for treating solid tumors.
The need for a CSR to add potency to T-cell function has been well described for decades. The combination of a CAR and CSR (with a B-cell maturation antigen and CD38 pair for treating myeloma, and a prostate-specific membrane antigen and prostate stem cell antigen pair for treating prostate cancer) has been previously proposed as a strategy to improve the specificity of CAR T-cell therapy.33,34 Another study combining transgenic TCRs specific for surviving with CD19 CAR T cells showed superior antitumor activity in B-cell malignancies than the TCR therapy alone, further demonstrating the importance of inclusion of costimulatory signal to CAR T cells.35 In contrast to our studies, these CAR T-cell constructs used traditional CD3 ζ-chain signaling whereas the AbTCR-CSR platform uses the γδTCR for the primary signaling domain. Another advance here is that the AbTCR-CSR platform targets 2 different prominent antigens, 1 intracellular antigen and 1 cell surface lineage antigen both expressed in AML cells that would vastly broaden the range of possible targets, with improved specificity.
In summary, ESK2 AbTCR-CSR T cells armed with 2 distinct receptors could be a potent and specific therapy that avoids myelosuppression and toxicity commonly seen in AML cell therapies. This approach could potentially be expanded into therapies for other cancer types.
Acknowledgments
The authors thank Besnik Qeriqi and Elisa De Stanchina for the assistance in animal studies, and Mei Hong and Sal Fuerstenberg for editing the manuscript.
A.K. is a Scholar of the Leukemia & Lymphoma Society. This work was supported by National Institutes of Health, National Cancer Institute grants RO1 CA55349, PO1 CA23766, P30 CA008748, R35 CA241894 (D.A.S.), and 1R50CA265328-01A1 (T.D.).
ARTEMIS is a registered trademark owned by Eureka Therapeutics Inc.
Authorship
Contribution: T.D., G.X., C.L., and D.A.S. conceptualized, developed the study, and wrote the manuscript; G.X., S.S.M., and J.M. performed all AbTCR-CSR experiments and analyzed data; J.X. and Z.C. engineered all formats of antibodies used in this study; A.Y.C. performed computational screening of off-target epitopes; T.K., C.J., and W.C. performed all experiments; H.L. and A.P. performed colony-forming unit assays; and A.D., S.Y., S.T., M.K., and A.K. provided clinical samples and helpful discussions.
Conflict-of-interest disclosure: D.A.S. serves on a board of, or has equity in, or income from, Lantheus, Sellas, Iovance, Pfizer, Actinium Pharmaceuticals, OncoPep, Repertoire, Sapience, Atengen, and Eureka Therapeutics. A.K. is a consultant for Novartis, Rgenta, and Blueprint Medicines. T.D. is a consultant for Eureka Therapeutics. A.D. has patents covering CD33 binders for CAR T cells and bispecifics and is on a board, or has equity, or income from NomoCan Pharmaceuticals and PromiCell Therapeutics Inc. The remaining authors declare no competing financial interests.
The current affiliation of A.Y.C. is Regeneron, Tarrytown, NY.
Correspondence: David A. Scheinberg, Molecular Pharmacology, Center for Experimental Therapeutics; Leukemia Service Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; email: scheinbd@mskcc.org.
References
Author notes
∗G.X., S.S.M., and J.M. contributed equally to this study.
Data are available on request from the corresponding author, David A. Scheinberg (scheinbd@mskcc.org).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal