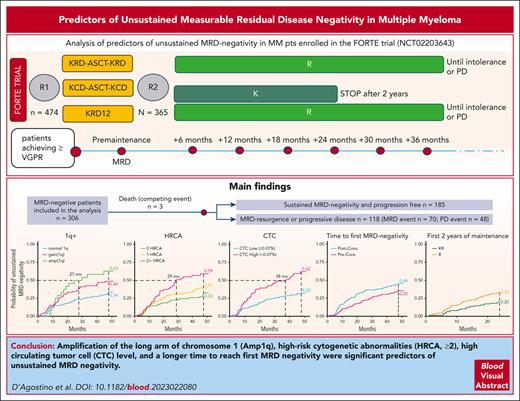
Amp(1q), HRCA ≥2, high CTC levels, and a longer time to reach first MRD negativity are significant predictors of unsustained MRD negativity.
Maintenance treatment with carfilzomib-lenalidomide reduces the risk of unsustained MRD negativity vs lenalidomide alone.
Visual Abstract
The prognostic impact of achieving and in particular maintaining measurable residual disease (MRD) negativity in multiple myeloma is now established; therefore, identifying among MRD-negative patients the ones at higher risk of losing MRD negativity is of importance. We analyzed predictors of unsustained MRD negativity in patients enrolled in the FORTE trial (NCT02203643). MRD was performed by multiparameter flow cytometry (sensitivity of 10−5) at premaintenance and every 6 months thereafter. The cumulative incidence (CI) of MRD resurgence and/or progression was analyzed in MRD-negative patients. A total of 306 of 474 (65%) MRD-negative patients were analyzed. After a median follow-up of 50.4 months from MRD negativity, 185 of 306 (60%) patients were still MRD negative and progression free, 118 (39%) lost their MRD-negative status, and 3 patients (1%) died without progression. Amp1q vs normal (4-year CI, 63% vs 34), ≥2 concomitant high-risk cytogenetic abnormalities vs 0 (4-year CI, 59% vs 33%), circulating tumor cells at baseline (high vs low at 4-year CI, 62% vs 32%), and time-to-reach MRD negativity postconsolidation vs preconsolidation (4-year CI, 46% vs 35%) were associated with a higher risk of unsustained MRD negativity in a multivariate Fine-Gray model. During the first 2 years of maintenance, patients receiving carfilzomib-lenalidomide vs lenalidomide alone had a lower risk of unsustained MRD negativity (4-year CI, 20% vs 33%).
Introduction
With highly effective multiple myeloma (MM) regimens, 50% to 80% of patients with MM achieve now bone marrow measurable residual disease (MRD) negativity.1-3
The challenge is therefore moving from achieving MRD negativity to sustaining it over time, as sustained MRD negativity is an even stronger predictor of long-term outcomes.4 Indeed, MRD reappearance usually predicts future relapses and worse outcomes,5 but few data are available about the time between MRD reappearance and relapse, as well as on factors predictive of MRD resurgence, including baseline risk features and treatment administered.
The aim of our analysis is to explore the impact of baseline risk features, treatment administered, and time to MRD achievement on the risk of losing MRD-negative status in MRD-negative patients enrolled in the FORTE clinical trial (NCT02203643).
Study design
FORTE trial6 details have been previously published and are described in the supplemental Methods (available on the Blood website).
All patients who achieved MRD negativity by multiparametric flow cytometry (sensitivity of 10−5) were included in the analysis. MRD was evaluated at suspected complete response, at premaintenance in patients achieving very good partial response or better, and then monitored every 6 months during maintenance. Severe hemodilution of the first MRD-negative sample was ruled out. The primary end point of our analysis was the cumulative incidence (CI) of MRD resurgence or progressive disease (PD) starting from the first MRD-negative evaluation. Secondary end points were to identify factors predictive of losing MRD-negative status over time in a multivariate Fine-Gray model. An MRD-positive result or PD, whichever comes first, was considered an event, whereas death without progression was considered a competing event. All presented data regarding predictive factors of unsustained MRD negativity are output of the multivariate model. Included variables are described in the supplemental Methods.
This trial, its protocol, and its amendments were approved by the ethics committees or institutional review boards at each of the participating centers. All patients gave written, informed consent before participating in the trial, which was done in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Results and discussion
A total of 310 of 474 (65%) enrolled patients achieved MRD negativity. Among MRD-negative patients, 4 were excluded because they went off trial soon after MRD negativity and no further MRD/response evaluation was available; thus, the analyzed population includes 306 patients. Patients’ characteristics are presented in supplemental Table 1. Median time to first MRD negativity was 8.9 months. After a median follow-up of 50.4 months from MRD negativity, 185 of 306 (60%) patients were still MRD negative and progression free, 118 of 306 (39%) lost their MRD-negative status, and 3 (1%) died without progression.
In patients who lost their MRD-negative status, a 1-year sustained MRD negativity was observed in 42 of 118 (36%) patients. Although we acknowledge that MRD resurgence in patients coming from a 1-year sustained MRD negativity may occur later and at lower rates, we still observe MRD resurgence and/or relapse in these patients, suggesting that 1 year between 2 MRD-negative evaluations could be not enough to identify patients without MRD resurgence and/or relapse.
Among patients with unsustained MRD negativity, 70 of 118 (59%) had a positive MRD test result in the bone marrow aspirate before PD, and 48 of 118 (41%) met criteria for PD before having a positive MRD test result in the bone marrow.
Among the 48 patients with PD before a positive MRD test result, 22 of 48 (46%) patients did not have an MRD assessment within the past 6 months, whereas among the remaining 26 patients with a recent MRD-negative assessment, 16 of 26 (62%) have skeletal/extramedullary relapse without biochemical PD.
Among the 70 patients with MRD resurgence before PD, median time from MRD positivity to conventional progression-free survival (PFS) event (PD or death) was 22.3 months, whereas median time from MRD positivity to next treatment was 34 months (supplemental Figure 1). This delay may be exploited to design strategies to reinduce MRD negativity.
In our analysis, International Staging System (ISS) (I vs II/III) and lactate dehydrogenase did not predict unsustained MRD negativity (supplemental Figure 2). ISS III patients represented only 13% of the whole MRD-negative population, and despite having a 4-year CI of unsustained MRD negativity of 52%, there was not a statistically significant difference compared with ISS I/II patients (P = .80). On the other hand, a trend toward higher risk of losing MRD negativity was present for patients with high-risk cytogenetic (del[17p] and/or t[4;14] and/or t[14;16]) vs standard risk (hazard ratio [HR], 1.54; P = .06; 4-year CI, 54% vs 36%) (supplemental Figure 2).
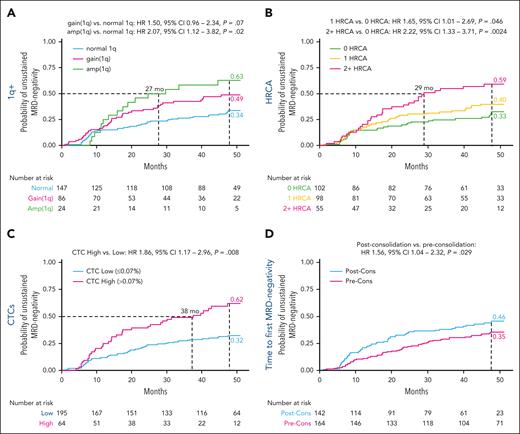
Regarding gain/amplification of chromosome 1q abnormalities, the presence of amplification(1q) and gain(1q) was associated with a higher risk of unsustained MRD negativity vs normal 1q (4-year CI, 63% vs 49% vs 34%, respectively). Specifically, amplification(1q) significantly increased the risk of unsustained MRD negativity (HR, 2.07; P = .02) (Figure 1A).
Predictors of unsustained MRD negativity. (A) Probability of unsustained MRD negativity in patients with amplification (amp)(1q) and gain(1q) and without 1q abnormalities. (B) Probability of unsustained MRD negativity in patients with 0, 1, and ≥2 concomitant HRCAs. (C) Probability of unsustained MRD negativity in patients with CTCs ≤0.07% vs >0.07%. (D) Probability of unsustained MRD negativity in patients who reached first MRD negativity preconsolidation (Pre-Cons) and postconsolidation (Post-Cons). HRCA defined as gain/amp of chromosome 1q (1q+) and/or t(4;14) and/or t(14;16) and/or del(17p) detected by fluorescence in situ hybridization.
Predictors of unsustained MRD negativity. (A) Probability of unsustained MRD negativity in patients with amplification (amp)(1q) and gain(1q) and without 1q abnormalities. (B) Probability of unsustained MRD negativity in patients with 0, 1, and ≥2 concomitant HRCAs. (C) Probability of unsustained MRD negativity in patients with CTCs ≤0.07% vs >0.07%. (D) Probability of unsustained MRD negativity in patients who reached first MRD negativity preconsolidation (Pre-Cons) and postconsolidation (Post-Cons). HRCA defined as gain/amp of chromosome 1q (1q+) and/or t(4;14) and/or t(14;16) and/or del(17p) detected by fluorescence in situ hybridization.
Including 1q in the definition of high-risk cytogenetic abnormalities (HRCAs), the presence of ≥2 HRCAs (HR, 2.22; P = .002 vs 0 HRCA) or 1 HRCA (HR, 1.65; P = .046 vs 0 HRCAs) was also associated with significantly higher risk of unsustained MRD negativity (4-year CI, 59% vs 40% vs 33% for ≥2 vs 1 vs 0 HRCA, respectively) (Figure 1B).
High circulating tumor cells (CTCs) at baseline (>0.07%)7 vs low CTCs predicted a significantly higher risk of unsustained MRD negativity (4-year CI, 62% vs 32%; HR, 1.86; P = .008) (Figure 1C). The role of CTCs was confirmed even using a lower cutoff (≥0.01%) to define subgroups, in line with other reports8 and the companion article (supplemental Figure 3).
Interestingly, the timing of first MRD negativity had an impact on the risk of MRD resurgence. Specifically, reaching first MRD negativity after the start of consolidation predicted a higher risk of unsustained MRD negativity vs preconsolidation (4-year CI, 46% vs 35%; HR, 1.56; P = .03) (Figure 1D).
Regarding premaintenance treatment, there was a trend toward a longer MRD-negativity duration in patients treated with carfilzomib-lenalidomide-dexamethasone (KRd) plus autologous stem cell transplantation (ASCT) vs carfilzomib-lenalidomide-dexamethasone for 12 cycles (KRd12) (4-year CI, 34% vs 43%; HR, 0.73; P = .17) or vs carfilzomib-cyclophosphamide-dexamethasone (KCd) plus ASCT (4-year CI, 44%; HR, 0.72; P = .16).
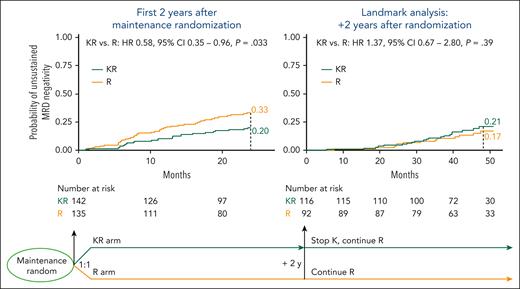
Regarding maintenance treatment, there was a trend toward a protective role of maintenance treatment with carfilzomib-lenalidomide (KR) vs maintenance treatment with lenalidomide alone (R) (4-year CI, 35% vs 43%; HR, 0.75; P = .15). However, because patients in the KR arm received carfilzomib only for the first 2 years of maintenance and then proceeded with lenalidomide alone, we performed 2 separate analyses taking into account the period from second randomization to carfilzomib discontinuation (first 2 years of maintenance) vs the period after carfilzomib discontinuation (landmark analysis after 2 years of maintenance).
In the first 2 years of maintenance, patients receiving KR vs R alone had a lower risk of unsustained MRD negativity (24-month CI, 20% vs 33%; HR, 0.58; P = .03), whereas the risk becomes superimposable in the landmark analysis after 2 years of treatment (4-year CI, 17% vs 21%; HR, 1.37; P = .39) (Figure 2).
Role of maintenance in unsustained MRD negativity. During the first 2 years after maintenance randomization (left), patients receiving KR maintenance vs R alone had a lower risk of unsustained MRD negativity (24-month CI, 20% vs 33%); this advantage is lost after the carfilzomib discontinuation (right, landmark analysis after 2 years of maintenance) (4-year CI, 17% vs 21%).
Role of maintenance in unsustained MRD negativity. During the first 2 years after maintenance randomization (left), patients receiving KR maintenance vs R alone had a lower risk of unsustained MRD negativity (24-month CI, 20% vs 33%); this advantage is lost after the carfilzomib discontinuation (right, landmark analysis after 2 years of maintenance) (4-year CI, 17% vs 21%).
Sustained MRD negativity is currently 1 of the most powerful prognostic factors in MM, and many trials exploring MRD-adapted strategies are ongoing.9 It is under evaluation if appropriate to deescalate/stop therapy in patients with sustained MRD negativity and if we should intensify treatment in high-risk patients or in patients with reappearance of MRD.
In this report, we saw that MRD-negative patients with no HRCA (including no Amp[1q]), low CTCs, and who turned MRD-negative early have low risk of MRD resurgence. In the companion article, the same observation on baseline CTCs and on the timing of MRD negativity achievement is provided in an independent cohort.
It may be appropriate to prospectively evaluate deintensification and even treatment interruption to reduce the risk of resistant clone selection and adverse events as infections and secondary primary malignant neoplasms.
The negative impact of ≥2 HRCAs reported in our analysis is in line with data from other trials. In the MASTER trial, patients with ≥2 HRCAs at baseline who discontinued treatment after 2 consecutive MRD-negative assessments presented a high risk of MRD resurgence and a lower PFS.3 A similar effect in patients with ≥2 HRCAs was observed also when therapy was given continuously, as in the GRIFFIN trial,10 suggesting that the biology of high-risk patients still plays a role despite the achievement of MRD negativity.
Strategies aiming to intensify therapy in MRD-positive patients may be appropriate also for MRD-negative patients at high risk of unsustained MRD negativity.
Indeed, in this report, we showed that a maintenance combination with carfilzomib and lenalidomide may reduce the risk of unsustained MRD negativity over lenalidomide maintenance alone, making it an appealing approach in high-risk patients even if MRD negative. Moreover, we also showed that the impact is present only during treatment, highlighting still the need for continuous therapy.
Once MRD positivity emerges, the need and the timing to start another line of treatment are still a matter of debate. We further analyzed the 70 patients with MRD resurgence before PD to find factors predicting progression and/or death or the need of next treatment after MRD resurgence. Results of a multivariate Cox model exploring PFS and time to next treatment from MRD resurgence are shown in supplemental Figure 4. With the limitation of low numbers of patients, a trend toward a higher risk was found in patients with Amp1q and ≥2 concomitant HRCAs, suggesting that these patients may be good candidates for an early change of treatment after MRD resurgence.
The results of our analysis, limited by the number of patients and the retrospective nature, should be validated in larger prospective cohorts of patients, including daratumumab-based combinations and new immune therapies. Our data were produced in the context of a transplant-eligible newly diagnosed population with MM; thus, the applicability of our results in the transplant-ineligible setting should be verified in a separate analysis.
Acknowledgments
The authors thank all the study participants, caregivers, physicians, nurses, and data managers of the participating centers. The authors were not precluded from accessing data in the study, and they accept responsibility to submit the manuscript for publication.
The UNITO-MM-01/FORTE trial was sponsored by the Università degli Studi di Torino (Italy), Department of Molecular Biotechnology and Health Sciences. Amgen and Celgene/Bristol Myers Squibb provided an unrestricted grant to conduct the trial, but had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or publication of this contribution. M.D. and F.G. were supported by a grant received from HORIZON-MISS-2021-CANCER-02–European research project ELMUMY.
Authorship
Contribution: All authors contributed to the acquisition, analysis, or interpretation of data for this work. All authors critically reviewed the work for important intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors had access to and verified the underlying data. M.D., G.B., S.O., C.V., B.B., and F.G. contributed to conceptualization, formal analysis, methods, and writing of the original draft. M.D. and G.B. contributed to visualization. B.B. and F.G. contributed to supervision. All authors contributed to data curation, investigation, resources, validation, and writing of the manuscript in terms of review and editing. All authors had access to all the data reported in the study and had final responsibility for the decision to submit this manuscript for publication.
Conflict-of-interest disclosure: M.D. has received honoraria from GlaxoSmithKline, Sanofi, and Janssen; and has served on the advisory boards for GlaxoSmithKline, Sanofi, and Bristol Myers Squibb. A.B. has served on the advisory boards for Janssen, Amgen, GlaxoSmithKline, and Pfizer. P.C. has received honoraria from AbbVie, ADC Therapeutics (data safety monitoring board), Amgen, Celgene, Daiichi Sankyo, Gilead/Kite, GSK, Incyte, Janssen, KyowaKirin, Nerviano Medical Science, Novartis, Pfzier, Roche, Sanofi, SOBI, and Takeda; and has received support for attending meetings and/or travel from AbbVie, Amgen, BMS, Celgene, Gilead/Kite, Janssen, Novartis, Roche, and Takeda. S.O. has received honoraria from Amgen, Celgene/Bristol Myers Squibb, and Janssen; and has served on the advisory boards for Adaptive Biotechnologies, Janssen, Amgen, and Takeda. A.G. has received honoraria from Janssen, Amgen, and Bristol Myers Squibb; and has received grant research from Janssen. B.G. has received honoraria from Janssen, Amgen, Bristol Myers Squibb, GlaxoSmithKline, Sanofi, and Takeda; and has served on the advisory boards for Janssen, Amgen, Bristol Myers Squibb, GlaxoSmithKline, Sanofi, and Takeda. K.M. has received honoraria from Janssen, Bristol Myers Squibb, Amgen, Sanofi, GlaxoSmithKline, Takeda, and Pfizer. L.P. has received honoraria from Celgene, Bristol Myers Squibb, Amgen, Takeda, and Janssen; has served on the advisory boards for Celgene, Bristol Myers Squibb, Amgen, and Janssen; and has received consultancy fees from Janssen. R.Z. has served on the advisory boards for Janssen, Bristol Myers Squibb, Amgen, Sanofi, GlaxoSmithKline, Menarini, and Oncopeptides. M.T.P. has received honoraria from Janssen, Bristol Myers Squibb, Amgen, Sanofi, GlaxoSmithKline, and Takeda; has served on the advisory boards for Janssen, Bristol Myers Squibb, Amgen, Sanofi, GlaxoSmithKline, Takeda, Roche, Oncopeptides, Pfizer, and Menarini; and has received support for attending meetings and/or travel from Janssen, Bristol-Myers Squibb, Amgen, Sanofi, and Takeda. B.B. has received honoraria from Amgen, Janssen, Novartis, BeiGene, Bristol Myers Squibb, GlaxoSmithKline, Jazz Pharmaceuticals, Astrazeneca, and Incyte; and has served on the advisory boards for Amgen and Jazz Pharmaceuticals. P.M. has received honoraria from Celgene, Janssen, Takeda, Bristol Myers Squibb, Amgen, Novartis, Gilead, Jazz, Sanofi, AbbVie, and GlaxoSmithKline; and has served on the advisory boards for Celgene, Janssen, Takeda, Bristol Myers Squibb, Amgen, Jazz, Sanofi, AbbVie, and GlaxoSmithKline. F.G. has received honoraria from Amgen, Celgene, Janssen, Takeda, Bristol Myers Squibb, AbbVie, and GlaxoSmithKline; and has served on the advisory boards for Amgen, Celgene, Janssen, Takeda, Bristol Myers Squibb, AbbVie, GlaxoSmithKline, Roche, Adaptive Biotechnologies, Oncopeptides, bluebird bio, and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Francesca Gay, Division of Hematology, Department of Molecular Biotechnology and Health Sciences, University of Torino, Turin, Italy, Division of Hematology, Azienda Ospedaliero-Universitaria Città della Salute e della Scienza di Torino, Via Genova 3, 10126 Turin, Italy; email: francesca.gay@unito.it.
References
Author notes
Data are available on request from the corresponding author, Francesca Gay (francesca.gay@unito.it). The sponsor of the UNITO-MM-01/FORTE trial, the University of Turin, via the corresponding author Francesca Gay, is responsible for evaluating and eventually accepting or refusing every request to disclose data and their related documents, in compliance with the ethical approval conditions, in compliance with applicable laws and regulations, and in conformance with the agreements in place with the involved subjects, the participating institutions, and all the other parties directly or indirectly involved in the participation, conduct, development, management, and evaluation of this analysis.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal