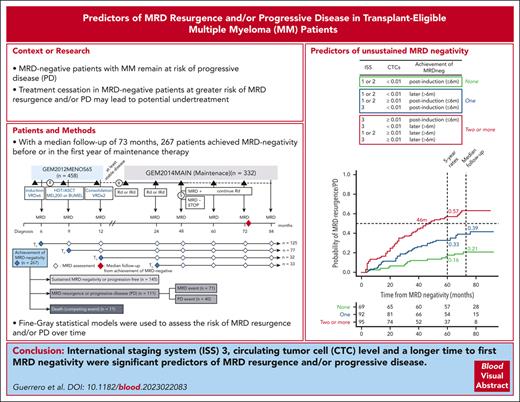
ISS 3, high CTC levels, and a longer time to first MRD negativity are significant predictors of MRD resurgence and/or progressive disease.
Visual Abstract
The role of measurable residual disease (MRD) negativity as a biomarker to stop treatment is being investigated in transplant-eligible patients with multiple myeloma (MM). Thus, it is important to identify risk factors of MRD resurgence and/or progressive disease (PD) among patients achieving undetectable MRD to avoid undertreating them. Here, we studied 267 newly diagnosed transplant-eligible patients with MM enrolled in the GEM2012MENOS65 and GEM2014MAIN clinical trials who achieved MRD negativity by next-generation flow cytometry. After a median follow-up of 73 months since the first MRD negative assessment, 111 of the 267 (42%) patients showed MRD resurgence and/or PD. The only prognostic factors at diagnosis that predicted MRD resurgence and/or PD were an International Staging System (ISS) 3 and the presence of ≥0.01% circulating tumor cells (CTCs). Failure to achieve MRD negativity after induction also predicted higher risk of MRD resurgence and/or PD. Patients having 0 vs 1 vs ≥2 risk factors (ISS 3, ≥0.01% CTCs, and late MRD negativity) showed 5-year rates of MRD resurgence and/or PD of 16%, 33%, and 57%, respectively (P < .001). Thus, these easily measurable risk factors could help refine the selection of patients for whom treatment cessation after MRD negativity is being investigated in clinical trials. This trial was registered at www.clinicaltrials.gov as NCT01916252 and NCT02406144.
Introduction
Patients with multiple myeloma (MM) achieving undetectable measurable residual disease (MRD) enjoy longer survival.1-5 As such, the role of MRD negativity as the end point for treatment duration and adaptation is being investigated in numerous clinical trials.6 This includes both the intensification and/or prolongation of therapy in transplant-eligible patients with persistent MRD, as well as treatment cessation in those showing sustained MRD negativity during maintenance.7-12 Notwithstanding the value of these investigations, MRD-negative patients remain at risk of progressive disease (PD), and the rates of progression at both early and late time points remain largely unknown, particularly in cases in which undetectable MRD has been defined using highly sensitive next-generation methods.13
Because of the increased use of MRD testing in current clinical trials, mounting evidence suggests that the MRD status of patients is more dynamic than previously described.14 More important, recent data have confirmed prior observations, establishing a clear correlation between MRD resurgence and poorer survival outcomes.15 Therefore, investigating treatment cessation in MRD-negative patients at greater risk of MRD resurgence and/or PD may lead to potential undertreatment. Because of this, we sought to develop a predictive model that accurately assesses the risk of MRD resurgence and/or PD in patients with MM who have achieved undetectable MRD by next-generation flow (NGF) cytometry.
Study design
Newly diagnosed and transplant-eligible patients with MM enrolled in the GEM2012MENOS65 and GEM2014MAIN clinical trials, who achieved undetectable MRD by NGF, were analyzed.16,17 The GEM2012MENOS65 is an open-label phase 3 trial in which 458 patients received 6 induction cycles of bortezomib, lenalidomide, and dexamethasone, followed by an autologous stem cell transplant (ASCT) conditioned with Bu-Mel or Mel-200 high-dose therapy (HDT), and 2 consolidation cycles of bortezomib, lenalidomide, and dexamethasone. Patients with at least minimal response were randomized in the GEM2014MAIN trial to receive maintenance with lenalidomide and dexamethasone (RD) or RD plus ixazomib for 2 years, after which patients continued with RD for 3 additional years if MRD positive or stopped therapy if MRD negative. PD was defined following the International Myeloma Working Group response criteria published in 2006. Each study site’s Independent Ethics Committee approved the protocol, and written informed consent forms were required before patient enrollment. Studies were conducted as per the ethical principles of the Declaration of Helsinki and were registered at www.clinicaltrials.gov with respective identifiers NCT01916252 and NCT02406144.
NGF cytometry was performed to assess circulating tumor cells (CTCs) in peripheral blood at diagnosis. Fluorescence in situ hybridization was performed in CD138+ immunomagnetically enriched bone marrow plasma cells tested for del(1p32), 1q21 gain or amplification, IgH translocations, and del(17p13). Patients were considered positive for any of these abnormalities if present in ≥10% of CD138+ plasma cells. Patients were classified as high risk according to the presence of t(4;14), t(14;16), and/or del(17p13). MRD was assessed following the EuroFlow18,19 NGF standard operation protocols in bone marrow aspirates collected after induction, HDT/ASCT, consolidation, and yearly during 5 years since initiation of maintenance therapy. Undetectable MRD was defined with a limit of detection of 2 × 10-6.
A multivariate Fine-Gray statistical model was applied to the data set, subsequently fitting a weighted Cox model to assess the cumulative incidence of MRD resurgence and/or PD.20 Death without PD was considered a competing event. Each patient was landmarked from the moment of achievement of undetectable MRD to avoid immortal time bias. Survival curves and hazard ratios were generated with R, version 4.2.1, package survival, version 3.4-0. Individual patient-level data are available to researchers who provide a methodologically sound proposal to the corresponding author.
Results and discussion
A total of 267 of 458 (58%) patients achieved MRD negativity by NGF after induction (n = 125), HDT/ASCT (n = 77), or consolidation (n = 32), or after the first year of maintenance therapy (n = 33), and were eligible for the analysis. The median time to first MRD-negative assessment was 9 months (interquartile range [IQR], 6-9 months). Median follow-up from first MRD-negative assessment was 73 months (IQR, 67-82 months). A description of patients’ characteristics is included in supplemental Table 1 (available on the Blood website). Baseline and posttreatment characteristics of patients eligible vs ineligible to this study are included in supplemental Table 2. Because of the prerequisite of undetectable MRD, patients being studied (n = 267) have lower rates of high-risk factors and better clinical outcomes than those not included (n = 191).
Of the 267 eligible patients, 145 (54%) remained progression free and had sustained MRD negativity until last time to follow up, whereas 111 of 267 (42%) patients had MRD resurgence (n = 71) and/or PD (n = 40), and 11 of 267 (4%) patients died without PD. Among the 40 patients who relapsed without previous MRD resurgence, 29 (72%) did not have an MRD assessment within the last year. These findings suggest that rather than a false-negative result, more frequent testing could have captured MRD resurgence before PD.
Baseline risk factors, such as the original and revised International Staging System (ISS), lactate dehydrogenase levels, cytogenetic alterations, and CTCs, as well as the time to achievement of first MRD negative assessment, were explored using univariate analyses (supplemental Table 1). Interestingly, an ISS 3, a high percentage of CTCs at diagnosis (both ≥0.01%21 and >0.07%22), and failure to achieve MRD negativity after induction were significantly associated with MRD resurgence and/or PD, whereas lactate dehydrogenase levels and the revised ISS were not. A high-risk cytogenetic status tended toward significance.
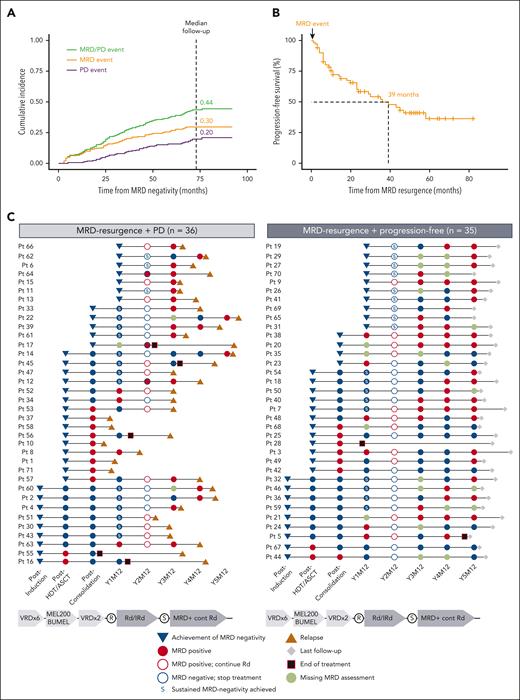
Being our aim the study of cumulative incidence of MRD reappearance and/or PD, we used a Fine-Gray statistical model to evaluate the risk of losing the MRD-negative status over time, where death without PD was considered a competing event. To characterize the clinical impact of the model, an MRD event was defined as MRD reappearance without PD, whereas a PD event was defined as clinical progression without previous MRD resurgence. Accordingly, the cumulative incidences of MRD and PD events were 0.30 and 0.20, respectively, with an overall cumulative incidence for both events of 0.44 (Figure 1A).
MRD resurgence and/or PD in patients (Pts) achieving undetectable MRD. (A) Cumulative incidence of MRD resurgence and PD events at the time of median follow-up (73 months from MRD negativity). (B) Median progression-free survival in patients with an MRD resurgence event. (C) Swimmer plots landmarked at the time of MRD-negative achievement, with subsequent MRD assessments and time to relapse or last follow-up. Patients with sustained MRD negativity before MRD resurgence (defined as MRD negative confirmed 1 year apart) are marked with an S. Patients having PD without previous MRD resurgence are represented in supplemental Figure 4. R, randomization.
MRD resurgence and/or PD in patients (Pts) achieving undetectable MRD. (A) Cumulative incidence of MRD resurgence and PD events at the time of median follow-up (73 months from MRD negativity). (B) Median progression-free survival in patients with an MRD resurgence event. (C) Swimmer plots landmarked at the time of MRD-negative achievement, with subsequent MRD assessments and time to relapse or last follow-up. Patients with sustained MRD negativity before MRD resurgence (defined as MRD negative confirmed 1 year apart) are marked with an S. Patients having PD without previous MRD resurgence are represented in supplemental Figure 4. R, randomization.
The median progression-free survival since MRD resurgence until PD or death was 39 months (Figure 1B). Time from achievement of first MRD negativity until MRD resurgence was similar in patients with or without subsequent disease progression (median, 21 [IQR, 8-34] and 25 [IQR, 13-47] months, respectively; P = .188) (Figure 1C). Accordingly, sustained MRD-negative rates (defined as MRD negativity confirmed in ≥2 samples analyzed ≥1 year apart) before MRD resurgence were similar in both groups of patients who had PD vs those who remained progression free (47% and 51%, respectively; Figure 1C). The value of 1-year sustained vs a single MRD-negative assessment is well established in clinical trials based on continuous therapy.11,23 However, it could be hypothesized that longer sustainability (eg, 3 years) could be required for a more individualized patient management, such as the fixed duration treatment approach used in the GEM2014MAIN trial.17
The rates of ISS 2 and 3 vs ISS 1 were significantly higher in the group of patients who relapse vs those who remained progression free after MRD resurgence (75% and 46%; P = .03) (supplemental Table 3). Of further interest, MRD levels at resurgence correlated with time to PD; patients displaying MRD levels <10-5, in between ≥10-5 and <10-3, and ≥10-3 showed median progression-free survival landmarked at the time of MRD resurgence of not reached, 39 and 6 months, respectively (supplemental Figure 1). These results highlight the importance of regular monitoring of not just MRD status, but also MRD levels, to evaluate efficacy and guide therapeutic decisions,14 such as early rescue intervention of patients with MRD resurgence in clinical trials.24
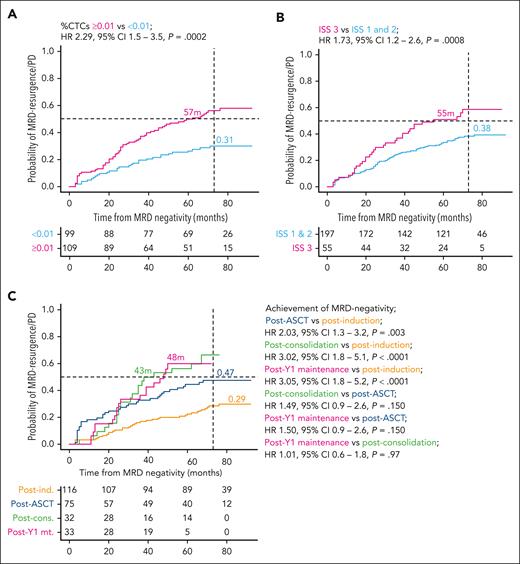
The Fine-Gray statistical model confirmed that increased percentages of CTCs at diagnosis (using both ≥0.01% and >0.07% thresholds) were significantly associated with a higher risk of MRD resurgence and/or PD (hazard ratio [HR], 2.29 [95% confidence interval {CI}, 1.5-3.5], P < .001; and HR, 1.95 [95% CI, 1.3-3.0], P = .002, respectively) (Figure 2A and supplemental Figure 2). Patients with ISS 3 had a 50% probability of MRD resurgence and/or PD at 55 months (HR, 1.73 [95% CI, 1.2 – 2.6], P = .008) (Figure 2B). High-risk cytogenetic abnormalities (HRCAs) (del[17p13], t[4;14], and t[14;16]) and 1q+ (gain and/or amplification) alterations did not identify patients at a higher risk of MRD resurgence and/or PD (supplemental Figure 3). However, the incorporation of 1q+ alterations into the combination of HRCAs showed a trend toward an adverse prognosis (HR, 1.43 [95% CI, 1.0-2.1], P = .079; supplemental Figure 3D). Only 4 patients had ≥2 HRCAs (del[17p13], t[4;14], and t[14;16]), and 3 of 4 showed MRD resurgence and/or PD. Patients who achieved MRD negativity after induction (<6 months) had significantly lower risk of MRD resurgence and/or PD than those who achieved MRD negativity at a later time point (Figure 2C). Of further interest, treatment randomization with Bu-Mel or Mel-200 HDT in the GEM2012MENOS65 trial, and with RD plus ixazomib vs RD in the GEM2014MAIN trial had no impact in the risk of MRD resurgence and/or PD (data not shown).
Fine-Gray analyses define predictors of unsustained MRD negativity. Patients were stratified according to (A) the percentage of CTCs at diagnosis defined per the cutoff ≥0.01%, (B) ISS 3 at baseline, and (C) time to achievement of MRD negativity. Accordingly, MRD was measured after induction (6 months since diagnosis), after ASCT (9 months), after consolidation (12-14 months), or after year 1 of maintenance therapy (24-26 months).
Fine-Gray analyses define predictors of unsustained MRD negativity. Patients were stratified according to (A) the percentage of CTCs at diagnosis defined per the cutoff ≥0.01%, (B) ISS 3 at baseline, and (C) time to achievement of MRD negativity. Accordingly, MRD was measured after induction (6 months since diagnosis), after ASCT (9 months), after consolidation (12-14 months), or after year 1 of maintenance therapy (24-26 months).
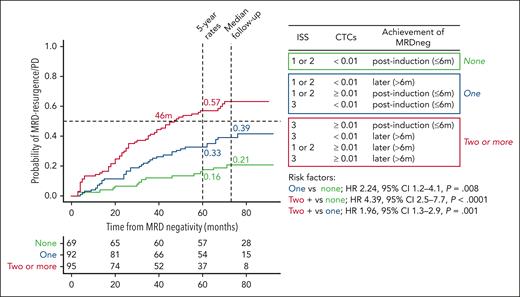
Because of the potential complementarity provided by patients’ ISS, CTC levels, and the time to achievement of first MRD-negative assessment, the 3 risk factors were modeled for a more accurate prediction of MRD resurgence and/or PD (Figure 3). Accordingly, patients having 0 vs 1 vs ≥2 risk factors showed 5-year rates of MRD resurgence and/or PD of 16%, 33%, and 57%, respectively (P < .001). This dynamic model relies on 3 easily measurable risk factors, and the insights it offers can be valuable in the careful selection of patients for whom treatment cessation is being contemplated because of MRD negativity.8,12,17,25 As with other prognostic models, its performance may vary according to the method being used (eg, cutoffs to define CTCs or the sensitivity of MRD negativity) and the treatment that patients receive. Accordingly, the findings from the FORTE trial uncovered how different induction and maintenance regimens may modulate the risk of MRD resurgence and/or PD among patients with undetectable MRD (companion article), as well as the emerging and reproducible role of CTCs in the risk stratification of MM.13,21,22 Additional validation is warranted to explore predictors of unsustained MRD negativity in different clinical scenarios.
Dynamic model to predict the risk of MRD resurgence and/or PD in patients achieving undetectable MRD. Patients are stratified into 3 groups according to the number of risk factors present at baseline (eg, ISS 3 and CTCs ≥0.01) and the time to achievement of MRD negativity (eg, after induction, eg, >6 months).
Dynamic model to predict the risk of MRD resurgence and/or PD in patients achieving undetectable MRD. Patients are stratified into 3 groups according to the number of risk factors present at baseline (eg, ISS 3 and CTCs ≥0.01) and the time to achievement of MRD negativity (eg, after induction, eg, >6 months).
Acknowledgments
The authors thank all investigators and participating centers of the GEM (Grupo Español de Mieloma)/PETHEMA (Programa para el Estudio de la Terapéutica en Hemopatías Malignas) cooperative study group. The authors acknowledge Alfonso Santiago and Carmen Carrero for the supportive administration by PETHEMA, as well as Roberto Maldonado and Arturo Touchard for data management.
This study was supported by grants from the Centro de Investigación Biomédica en Red–Área de Oncología–Instituto de Salud Carlos III and cofinanced by European Regional Development Funds (FEDER) (Centro de Investigacion Biomé [CIBER-ONC]); CB16/12/00369, CB16/12/00400, and CB16/12/00284), Instituto de Salud Carlos III/Subdirección General de Investigación Sanitaria, and cofinanced by FEDER funds (Formación en Investigación en Salud [FIS] numbers PI15/01956, PI15/02049, PI15/02062, PI18/01709, and PI19/01451), Instituto de Salud Carlos III/Subdirección General de Investigación Sanitaria, cofinanced by the European Social Fund Plus (FSE+) and the European Union (PFIS number FI21/00293), the Cancer Research UK (C355/A26819), FCAECC, and Fondazione AIRC per la Ricerca sul Cancro under the Accelerator Award Programme (EDITOR), the Black Swan Research Initiative of the International Myeloma Foundation, and the European Research Council Starting Grant 2015 under the MYELOMANEXT project (grant agreement 680200), the Cancer Research Innovation Spain Cancer Foundation (PR_EX_2020-02), and the European Union Horizon Europe Research and Innovation Programme under the ELMUMY project (grant agreement 101097094). This study was supported by the Riney Family Multiple Myeloma Research Program Fund and the International Myeloma Society.
Authorship
Contribution: C.G., J.F.S.-M., and B.P were responsible for study conception and design; N.P., M.-T.C., M.-J.C., N.C.G., M.F., A. Oriol, R.R., M.-T.H., R.M.-M., J. Bargay, F.d.A., L.P., A.P.G.-R., M.-S.G.-P., A. Orfao, M.-V.M, J.M.-L., L.R., J. Bladé, J.-J.L., and J.F.S.-M. provided study material and/or patients; C.G., J.F.S.-M., and B.P. analyzed and interpreted data; C.G., J.F.S.-M., and B.P. wrote the manuscript; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: N.P. reports honoraria from Amgen, Celgene, Janssen, Takeda, and The Binding Site; consulting or advisory role for Amgen, Celgene, Janssen, and Takeda; speakers' bureau for Celgene; research funding from Celgene, Janssen, Amgen, and Takeda; and travel, accommodations, and expenses from Amgen, Celgene, Janssen, and Takeda. M.-T.C. reports honoraria from Janssen, Celgene, and Abbvie. A.P.G.-R. reports honoraria from Janssen, Celgene, Takeda, and Amgen. L.R. reports honoraria from Janssen, Celgene, Amgen, and Takeda. J.M.-L. reports consulting or advisory role for Amgen, Celgene, Janssen, Takeda, Novartis, Incyte, and Adaptive; and research funding from BMS, Roche, and Janssen. M.-V.M. reports honoraria and membership on an entity’s Board of Directors or advisory committees for Janssen, Celgene, Takeda, Amgen, Adaptive, GSK, Sanofi, and Oncopeptides; and honoraria from membership in Board of Directors or advisory committees for Abbvie, Roche, Pfizer, Regeneron, and Seattle Genetics. J.-J.L. reports consulting or advisory role for Celgene, Takeda, Amgen, Janssen, and Sanofi; and travel accommodations and expenses from Celgene. J. Bladé reports honoraria from Janssen, Celgene, Takeda, Amgen, and Oncopeptides. J.F.S.-M. reports consultancy or advisory role for Abbvie, Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Karyopharm, MSD, Novartis, Roche, Sanofi, SecuraBio, and Takeda. B.P. reports honoraria for lectures from and membership on advisory boards with Adaptive, Amgen, Bristol Myers Squibb-Celgene, Janssen, Kite Pharma, Sanofi, and Takeda; unrestricted grants from Celgene, EngMab, Roche, Sanofi, and Takeda; and consultancy for Bristol Myers Squibb-Celgene, Janssen, and Sanofi. The remaining authors declare no competing financial interests.
A list of the investigators in the GEM/PETHEMA cooperative study group appears in the supplemental File.
Correspondence: Bruno Paiva, Clínica Universidad de Navarra, Centro de Investigación Médica Aplicada (CIMA), Av. Pío XII 55, 31008 Pamplona, Spain; email: bpaiva@unav.es.
References
Author notes
Individual patient-level data are available to researchers who provide a methodologically sound proposal to the corresponding author.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal