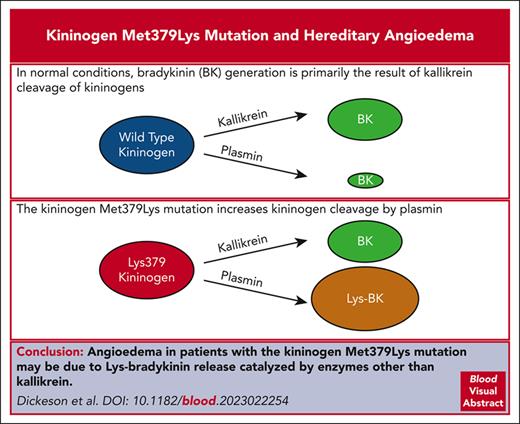
A Met379 to lysine substitution in high– and low–molecular weight kininogen is linked to hereditary angioedema.
The mutation increases HK and LK susceptibility to cleavage by plasmin, resulting in release of Lys-bradykinin (kallidin).
Visual Abstract
Hereditary angioedema (HAE) is associated with episodic kinin-induced swelling of the skin and mucosal membranes. Most patients with HAE have low plasma C1-inhibitor activity, leading to increased generation of the protease plasma kallikrein (PKa) and excessive release of the nanopeptide bradykinin from high-molecular-weight kininogen (HK). However, disease-causing mutations in at least 10% of patients with HAE appear to involve genes for proteins other than C1-inhibitor. A point mutation in the Kng1 gene encoding HK and low–molecular weight kininogen (LK) was identified recently in a family with HAE. The mutation changes a methionine (Met379) to lysine (Lys379) in both proteins. Met379 is adjacent to the Lys380-Arg381 cleavage site at the N-terminus of the bradykinin peptide. Recombinant wild-type (Met379) and variant (Lys379) versions of HK and LK were expressed in HEK293 cells. PKa-catalyzed kinin release from HK and LK was not affected by the Lys379 substitutions. However, kinin release from HK-Lys379 and LK-Lys379 catalyzed by the fibrinolytic protease plasmin was substantially greater than from wild-type HK-Met379 and LK-Met379. Increased kinin release was evident when fibrinolysis was induced in plasma containing HK-Lys379 or LK-Lys379 compared with plasma containing wild-type HK or LK. Mass spectrometry revealed that the kinin released from wild-type and variant kininogens by PKa is bradykinin. Plasmin also released bradykinin from wild-type kininogens but cleaved HK-Lys379 and LK-Lys379 after Lys379 rather than Lys380, releasing the decapeptide Lys-bradykinin (kallidin). The Met379Lys substitutions make HK and LK better plasmin substrates, reinforcing the relationship between fibrinolysis and kinin generation.
Introduction
Patients with the disorder hereditary angioedema (HAE) experience recurring bouts of soft tissue swelling primarily involving the oropharyngeal mucosa, subcutaneous tissues of the face and hands, genitals, and gastrointestinal tract.1-4 In most cases, angioedema is due to excessive formation of the vasoactive peptide bradykinin because of increased activity of the plasma kallikrein-kinin system (KKS).1,3,5 The KKS is composed of the zymogens prekallikrein (PK) and factor XII (FXII) and the cofactor/substrate high–molecular weight kininogen (HK).6-8 PK and FXII reciprocally convert each other to the proteases plasma kallikrein (PKa) and FXIIa.9-11 PKa then cleaves HK after Lys380 and Arg389 to release bradykinin.6-9 The physiologic effects of bradykinin are mediated primarily through specific bradykinin receptors expressed in many tissues.12,13 Basal bradykinin generation likely contributes to setting blood vessel permeability and tone,14,15 whereas higher concentrations at injury sites promote edema and pain sensation.6-8
PKa and FXIIa activity is regulated by the serpin C1-inhibitor (C1-INH).1-3,16 In most patients with HAE, KKS dysregulation is caused by reduced plasma C1-INH activity (5%-30% of normal activity).1-3,17 However, at least 10% of patients with HAE have normal plasma C1-INH activity (referred to as HAEnC1).3,18-21 Several mutations in genes unrelated to C1-INH have been identified in such individuals. A Thr328Lys/Arg substitution in FXII and a Lys330Glu replacement in plasminogen are the most common,18-24 and mechanisms have been proposed for how they may contribute to disease.10,25-28 In 2019, Bork et al described a mutation in the Kng1 gene in several members of a family.29 The mutation segregated with signs and symptoms of HAE in a manner indicating a high probability that it is causative. Kng1 encodes messenger RNAs for HK and the related plasma protein low–molecular weight kininogen (LK). The mutation causes Met379 in both kininogens to be replaced with Lys. The substitution is adjacent to Lys380 at one of the sites cleaved by PKa to release bradykinin from HK, suggesting that it may alter bradykinin release from HK by PKa. Alternatively, the basic Lys379 residue may create a novel cleavage site for PKa or other trypsin-like proteases that would result in release of the decapeptide Lys-bradykinin (kallidin), which also interacts with bradykinin receptors.13,30 We introduced Lys379 into human HK and LK, and studied the effects on kinin formation in purified protein and plasma systems.
Methods
Materials
Hemagglutinin (HA) epitope tag antibody agarose conjugate (Fisher Scientific, Atlanta, GA); human plasma–derived FIXa, FXa, FXIa, FXII, FXIIa, PK, and PKa (Enzyme Research Laboratories, South Bend, IN); α-thrombin and plasmin (Prolytix, Essex Junction, VT); S2302 (H-d-prolyl-l-phenylalanyl-l-arginine-p-nitroanilide dihydrochloride; DiaPharma, West Chester, OH); silica nanospheres (100 mm; nanoComposix, San Diego, CA); bradykinin enzyme-linked immunosorbent assay (ELISA) kit (Enzo Life Sciences, Farmingdale, NY); bradykinin standard (Eurofins DiscoveRx, Fremont, CA); Lys-bradykinin standard (Peptide Institute, Osaka, Japan). Captopril (Sigma-Aldrich, St Louis, MO); PKa inhibitor KV999272 (formerly VA999272) has been described previously31; tissue plasminogen activator (tPA; Alteplase, Genentech, South San Francisco, CA; normal human plasma (Precision BioLogic, Dartmouth, NS, Canada); human plasmas lacking HK and LK (George King Biomed); and PTT-A reagent (Diagnostica Stago, Asnieres-sur-Seine, France) were used in this study and sourced as indicated.
Recombinant proteins
The amino acid numbering system used in this manuscript for human HK, LK, FXII, and plasminogen assigns the number 1 to the initiator Met on the signal peptide, according to current international standards. The table in supplemental Figure 1, available on the Blood website, lists corresponding amino acid numbering based on assigning the number 1 to the first amino acid of the mature protein, to facilitate comparing results to older literature.
Kininogens
Complementary DNAs (cDNA) encoding wild-type human HK and LK (HK-Met379 and LK-Met379, respectively) in the expression vector pJVCMV were modified by adding a HA tag (Tyr-Pro-Tyr-Asp-Val-Pro-Asp-Tyr-Ala) to the C-termini.27 Site-directed mutagenesis was performed to alter the sequence encoding Met379 in wild-type HK and LK (HK-Met379 and LK-Met379) cDNAs to Lys to create the variants HK-Lys379 and LK-Lys379, respectively. All cDNAs underwent sequencing to verify the proper sequence. Kininogens were expressed in HEK293 cells and purified by chromatography on anti-HA agarose, as previously reported.27
Plasminogen
Human Glu-plasminogen was expressed in Expi293 cells (Fisher Scientific), concentrated from conditioned media on Lys-sepharose, and purified by ion exchange and size exclusion chromatography, as previously described.27
Chromogenic assay
Assays were performed in polyethylene glycol (PEG)-20000–coated microtiter plates. Assays for reciprocal activation of FXII (25 nM) and PK (60 nM) with or without HK (60 nM) were performed in 100 μL standard buffer (20 mM N-2-hydroxyethylpiperazine-N9-2-ethanesulfonic acid [pH 7.4], 100 mM NaCl, 0.1% PEG-8000, and 10 mM ZnCl2) containing 200 mM S-2302. In some reactions, 25 μg/mL silica nanospheres were added. Changes in optical density at 405 nm at 37°C were continuously monitored.
Kinin assay
For purified protein assays, HK-Met379, LK-Met379, HK-Lys379, and LK-Lys379 (200 nM) were incubated with PKa (2 nM for reactions containing HK; 50 nM for LK), plasmin (40 nM), thrombin (1 μM), FIXa (20 nM), FXa (20 nM), FXIa, (30 nM), or FXIIa (375 nM) in 150 μL reaction buffer (tris(hydroxymethyl)aminomethane [Tris]-buffered saline: 20 mM Tris-HCl, 136 mM NaCl; pH7.4) at 37°C. At various time points, 10 μL volumes were transferred into 90 μL ice-cold ethanol and placed at −80°C overnight. Samples underwent clarification by centrifugation at 10 000g for 1 hour. For all reactions, kinins were measured by ELISA (Enzo Life Sciences, Inc). For bradykinin release experiments, vehicle refers to 20 mM Tris-HCl and 136 mM NaCl, at pH7.4. Bradykinin and Lys-bradykinin were used to create standard curves.
For plasma assays, normal plasma was supplemented with captopril (500 μM), KV999272 (10 μM), and plasminogen (600 nM). After a 5-minute incubation, HK or LK (wild-type or variant), were added to 100 nM. Reactions (40 μL volumes) were started with tPA (125 nM) at 37°C. At various times, 3-μL reaction volumes were transferred to 12 μL ice-cold ethanol. Samples were clarified by centrifugation at 2000g for 10 minutes. Kinin release was determined as described earlier.
Mass spectrometry
Purified kininogen samples were prepared in the same manner as for the kinin ELISA. For all analytes, ionization was achieved using matrix-assisted laser desorption ionization (MALDI). Analytes were deposited with matrix on MALDI targets. Matrix, 2,5-dihydroxybenzoic acid (Sigma), was purified by recrystallization from hot water. The matrix solution was prepared with recrystallized 2,5-dihydroxybenzoic acid (15 mg/dL) in a mixture of water, acetonitrile, and formic acid at a ratio of 1:1:0.1. Matrix solution (1 μL) was spotted onto the MALDI target with 1 μL analyte sample and allowed to dry at room temperature. Samples were analyzed in a Bruker AutoFlex (Billerica, MA) mass spectrometer. Mass spectrometry data were collected in positive-ion reflectron mode, with a mass-to-charge (m/z) range of 600 to 6000. Spectra are from an average of at least 100 laser shots per sample spot. Mass spectra were analyzed with Compass 1.4 Flex Series software (Bruker Daltonics).
To determine the nature of an apparent 16-dalton increase in molecular mass for bradykinin and Lys-bradykinin observed in some experiments, tandem mass spectrometry measurements were collected on a Bruker SolariX XR 12 T Fourier Transform Ion Cyclotron Resonance mass spectrometer equipped with a dual electron spray ionization (ESI)/MALDI source (Bremen, Germany). Mass spectra were collected in positive mode between 98 and 2000 m/z with 2 million data points and a 0.5592-second transient time. Time of flight was set at 0.8 milliseconds; 24 to 48 scans were averaged per spectrum. Tandem mass spectrometry was performed using collision-induced dissociation with collision energies ranging from 30 to 50 V.
Mass calibration was performed using sodium trifluoro acetate (1 mg/mL in 50:50::MeOH:H2O) using ESI. Peptide spectra were acquired using MALDI and ESI. For the MALDI experiments, the laser power was set to a range from 50% to 60%, the laser frequency was 200 Hz, and between 5 and 350 laser shots were used, depending on the amount of signal. For the ESI experiments, samples were infused at a rate of 2 μL/min. The capillary inlet voltage was set to 4500 V with a −500 V end-plate offset. The nebulizer gas was set at 0.5 L/min, and the drying gas was set to 4 L/min at 180°C.
Results
Recombinant kininogens
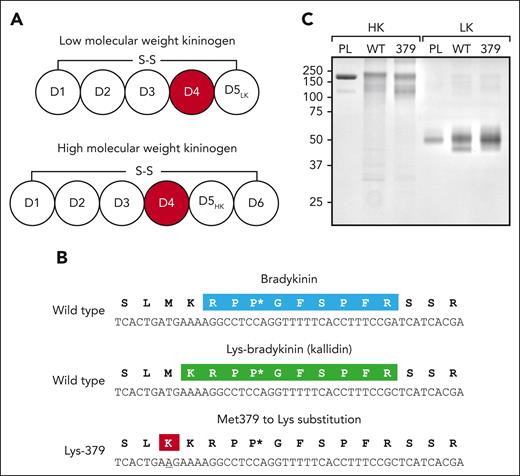
HK and LK are encoded by alternatively spliced messenger RNAs from the Kng1 gene.32-35 Both proteins have D1, D2, D3, and D4 domains (Figure 1A). D4 contains the bradykinin (Figure 1B, top) and Lys-bradykinin (Figure 1B, middle) sequences. The HK D5 domain mediates surface binding,36 and the HK D6 domain binds to PK or FXI,37 allowing HK to function as a cofactor during surface-induced contact activation (Figure 1A). LK lacks D6, and its shorter D5 domain probably does not support surface binding. PKa cleaves wild-type HK after Lys380 and Arg389 (Figure 1B, top), releasing bradykinin. If a kininogen amino acid numbering system that assigns the number 1 to the first residue of the mature kininogens found in plasma is used, then these residues are designated Lys362 and Arg371, respectively (supplemental Figure 1). PKa cleaves wild-type LK at the same sites, although LK is a poor PKa substrate, probably because it lacks the PKa–binding site on the D6 domain. LK is thought to be primarily a substrate for tissue kallikreins, which cleave it after Met379 and Arg389 to release Lys-bradykinin.38,39 The position of the Met379Lys substitution in variant HK and LK is shown in the last row of Figure 1B. The residue corresponding to amino acid 379 is Met in most mammalian kininogens and is a hydrophobic amino acid in nearly all (supplemental Figure 2).
Recombinant kininogen. (A) Schematic diagrams of human LK (top) and HK (bottom) showing domain structure and the disulfide bond connecting the N- and C-termini. The bradykinin sequence is contained within the common D4 domain. (B) Sequence of bradykinin (top; light blue) and kallidin (middle; green) in wild-type (WT) kininogens. Also shown is the position of Lys at position 379 in the HK and LK variants HK-Lys379 and LK-Lys379, respectively (bottom; red). The asterisk indicates a proline residue (Pro383) that becomes oxidized during expression or purification of the recombinant kininogens (supplemental Figure 5). (C) Sodium dodecyl sulfate– polyacrylamide gel electrophoresis of purified plasma-derived and recombinant kininogens. Samples (2 μg) of plasma-derived HK or LK (PL), recombinant HK/LK-Met379 (WT), and recombinant HK/LK-Lys379 (379) underwent electrophoresis on an 8% polyacrylamide nonreducing gel, followed by staining with Coomassie blue. Positions of the molecular mass standard in kilodaltons are shown at the left of the figure.
Recombinant kininogen. (A) Schematic diagrams of human LK (top) and HK (bottom) showing domain structure and the disulfide bond connecting the N- and C-termini. The bradykinin sequence is contained within the common D4 domain. (B) Sequence of bradykinin (top; light blue) and kallidin (middle; green) in wild-type (WT) kininogens. Also shown is the position of Lys at position 379 in the HK and LK variants HK-Lys379 and LK-Lys379, respectively (bottom; red). The asterisk indicates a proline residue (Pro383) that becomes oxidized during expression or purification of the recombinant kininogens (supplemental Figure 5). (C) Sodium dodecyl sulfate– polyacrylamide gel electrophoresis of purified plasma-derived and recombinant kininogens. Samples (2 μg) of plasma-derived HK or LK (PL), recombinant HK/LK-Met379 (WT), and recombinant HK/LK-Lys379 (379) underwent electrophoresis on an 8% polyacrylamide nonreducing gel, followed by staining with Coomassie blue. Positions of the molecular mass standard in kilodaltons are shown at the left of the figure.
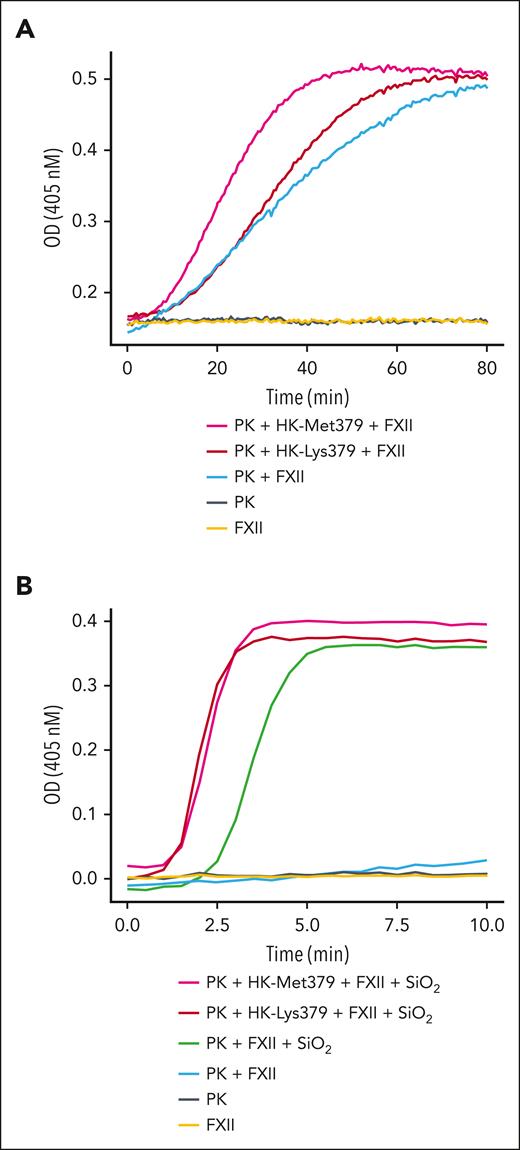
Recombinant wild-type (HK-Met379 and LK-Met379) and variant (HK-Lys379 and LK-Lys379) kininogens were expressed in HEK293 cells and purified from conditioned media (Figure 1C). When PK and FXII are mixed in solution in the absence of a surface they reciprocally convert each other to the proteases PKa and FXIIa (Figure 2A).9-11 In such reactions, HK-Met379 enhanced the rate of reciprocal activation modestly, whereas HK-Lys379 had little effect. This indicates that the Lys379 substitution is unlikely to cause HAE by increasing basal reciprocal activation of PK and FXII. When silicate nanospheres were added to reactions to induce contact activation, HK-Met379 and HK-Lys379 enhanced reciprocal activation comparably, indicating that the cofactor activity of the variant is intact. (Figure 2B).
Reciprocal activation of PK and FXII. (A) Reciprocal activation of PK (60 nM) and FXII (25 nM) in the presence of HK-Met379 (magenta; 60 nM), HK-Lys379 (red; 60 nM) or in the absence of HK (light blue) was assessed with a chromogenic substrate assay. Cleavage of S-2302 (200 mM) was followed by continuous changes in optical density (OD; 405 nm) on a spectrophotometer. Control reactions that contained only PK (steel blue) or FXII (gold) are also shown. (B) Reciprocal activation of PK and FXII was performed as described earlier but reactions also contained silica nanospheres (25 mg/mL; 100 nm average diameter).
Reciprocal activation of PK and FXII. (A) Reciprocal activation of PK (60 nM) and FXII (25 nM) in the presence of HK-Met379 (magenta; 60 nM), HK-Lys379 (red; 60 nM) or in the absence of HK (light blue) was assessed with a chromogenic substrate assay. Cleavage of S-2302 (200 mM) was followed by continuous changes in optical density (OD; 405 nm) on a spectrophotometer. Control reactions that contained only PK (steel blue) or FXII (gold) are also shown. (B) Reciprocal activation of PK and FXII was performed as described earlier but reactions also contained silica nanospheres (25 mg/mL; 100 nm average diameter).
Kinin generation mediated by PKa
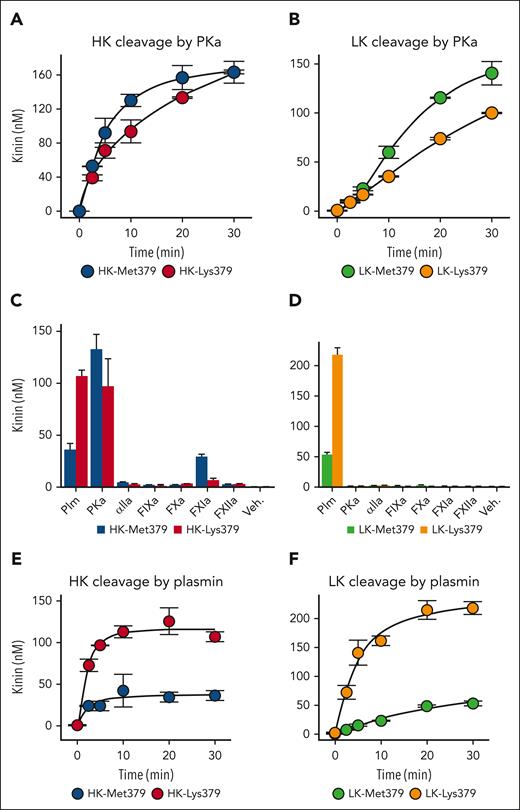
PKa-mediated kinin release from HK was measured by ELISA (Figure 3A). The rate of release was comparable for HK-Met379 and HK-Lys379, indicating that the Lys379 substitution does not alter PKa-mediated HK cleavage. A higher PKa concentration is required to release detectable bradykinin from LK.27 In such reactions, the rate of kinin release was slightly greater with wild-type LK-Met379 than with HK-Lys379 (Figure 3B). These findings suggest that PKa is not responsible for excess kinin generation in patients carrying the kininogen Lys379 substitution.
Kinin generation from recombinant HK/LK-Met379 and HK/LK-Lys379. For all reactions, the kininogen concentration was 200 nM. (A) Time courses of kinin formation for HK-Met379 (blue) or HK-Lys379 (red) incubated with 2 nM PKa and (B) LK-Met379 (light green), or LK-Lys379 (yellow) incubated with 50 nM PKa. (C) Kinin formed from HK-Met379 (blue) or HK-Lys379 (red); and (D) LK-Met379 (light green) or LK-Lys379 (yellow) incubated for 30 minutes with 40 nM plasmin (Plm), 2 nM kallikrein (PKa), 1000 nM α-thrombin (αIIa), 20 nM FIXa, 20 nM FXa, 30 nM FXIa (or 25 nM FXIIa); vehicle (Veh.) is Tris-buffered saline with no added enzyme. (E) Time courses of HK-Met379 (blue) or HK-Lys379 (red); and (F) LK-Met379 (light green) or LK-Lys379 (yellow) incubated with 40 nM plasmin. For all reactions kinin generation was determined by ELISA, and results shown are means ± 1 standard deviation for 2 runs.
Kinin generation from recombinant HK/LK-Met379 and HK/LK-Lys379. For all reactions, the kininogen concentration was 200 nM. (A) Time courses of kinin formation for HK-Met379 (blue) or HK-Lys379 (red) incubated with 2 nM PKa and (B) LK-Met379 (light green), or LK-Lys379 (yellow) incubated with 50 nM PKa. (C) Kinin formed from HK-Met379 (blue) or HK-Lys379 (red); and (D) LK-Met379 (light green) or LK-Lys379 (yellow) incubated for 30 minutes with 40 nM plasmin (Plm), 2 nM kallikrein (PKa), 1000 nM α-thrombin (αIIa), 20 nM FIXa, 20 nM FXa, 30 nM FXIa (or 25 nM FXIIa); vehicle (Veh.) is Tris-buffered saline with no added enzyme. (E) Time courses of HK-Met379 (blue) or HK-Lys379 (red); and (F) LK-Met379 (light green) or LK-Lys379 (yellow) incubated with 40 nM plasmin. For all reactions kinin generation was determined by ELISA, and results shown are means ± 1 standard deviation for 2 runs.
Cleavage of HK and LK by other proteases
The Lys379 substitution could create a new cleavage site for trypsin-like serine proteases. Several plasma serine proteases involved in coagulation were tested for their capacities to release kinins from recombinant HKs (Figure 3C) and LKs (Figure 3D). In these reactions, the kininogen concentration was held constant, but protease concentrations varied. FXIa, a homolog of PKa, cleaves HK-Met379 to release bradykinin, although at a slower rate than does PKa.27,39,40 Kinin release from HK-Lys379 by FXIa (30 nM) was slower than from HK-Met379 (Figure 3C). The KKS protease FXIIa (375 nM) did not release kinins from either HK-Met379 or HK-Lys379 (Figure 3C). The proteases thrombin (1000 nM), FXa (20 nM), and FIXa (20 nM) cleave HK-Lys379 but not HK-Met379, (supplemental Figure 3); however, this did not translate into kinin release (Figure 3C).
Several groups reported that the fibrinolytic serine protease plasmin cleaves HK.27,41-43 Plasmin-catalyzed kinin generation was greater from both HK-Lys379 and LK-Lys379 than from their wild-type counterparts (Figures 3C-D). The initial rate of kinin release from HK-Lys379 was 3.3-fold greater than from HK-Met379, but this does not tell the whole story. Previously, we observed that plasmin-catalyzed bradykinin release from HK appears to be stoichiometric, with the maximum release mirroring the plasmin concentration.27 This may reflect the formation of a tight interaction between HK and the plasmin kringle 5 domain.44 Results for HK-Met379 in Figure 3E are consistent with earlier results, but HK-Lys379 cleavage by plasmin does not follow this pattern, with kinin generation exceeding plasmin concentration (Figure 3E). The initial rate of kinin release from LK-Lys379 catalyzed by plasmin was ∼13-fold faster than with LK-Met379 (Figure 3F). These results suggest that the Lys379 substitution either alters cleavage of kininogens by plasmin after residue Lys380 (increasing bradykinin production) or creates a novel cleavage site for plasmin that would result in Lys-bradykinin release.
Mass spectrometry analysis of kininogens cleaved by PKa and plasmin
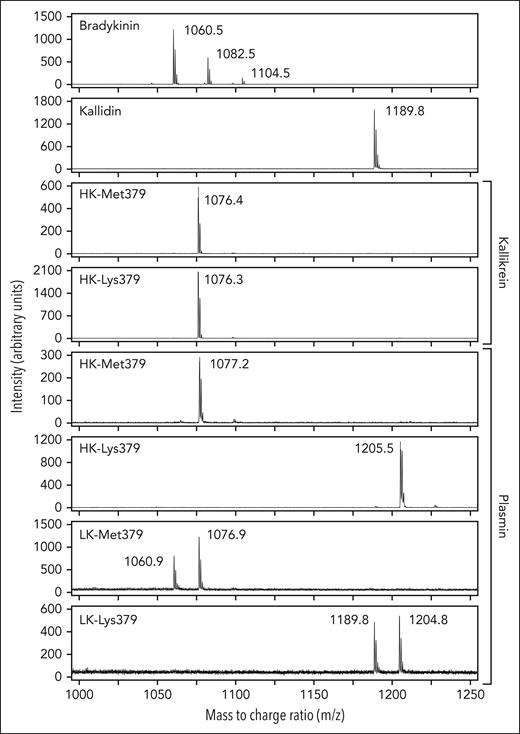
The ELISA we used to measure kinin release does not discriminate between bradykinin and Lys-bradykinin and is equally sensitive to both (supplemental Figure 4). It may recognize other kinins as well. We used mass spectrometry to characterize the kinins released by PKa and plasmin from recombinant kininogens. Previously, we showed that incubating plasma-derived HK with PKa or plasmin generated a peak with the same m/z ratio as a bradykinin standard (m/z = 1060-1061).27 With recombinant HK-Met379 and HK-Lys379, peaks obtained (m/z = 1076.4 and m/z = 1077.2, respectively; Figure 4) were ∼16 m/z units larger than with the bradykinin standard (m/z = 1060.5). Using tandem mass spectrometry (supplemental Figure 4), we determined that the peak represented bradykinin with replacement of a hydrogen atom with a hydroxyl group on the third amino acid in the bradykinin sequence (Pro383; Figure 1B). Modifications of prolines and other amino acids in this manner are relatively common and, in the case of the recombinant kininogens, likely occurred in cell culture or during protein purification.
Mass spectrometry. Mass spectrometry (MALDI) analysis of kinins generated by cleavage of WT HK/LK-Met379 and variant HK/LK-Lys379 kininogens (200 nM) by PKa or plasmin. The top 2 elution profiles show results for synthetic bradykinin and kallidin control peptides. For bradykinin, the peak at m/z = 1060.5 is unmodified bradykinin. Additional peaks are likely monosodated and disodated forms of bradykinin. For kallidin, the peak at m/z = 1189.8 is unmodified kallidin. When HK-Met379 and HK-Lys379 are digested with 2 nM kallikrein, peaks with m/z = 1076 were obtained, corresponding to an oxidized form of bradykinin (supplemental Figure 5). Similarly, when HK-Met379 and LK-Met379 were incubated with 40 nM plasmin, peaks with m/z = 1077.2 and m/z = 1076.9, respectively, representing oxidized bradykinin, were obtained. The peak with m/z = 1060.9 for LK-Met379 with plasmin represents unoxidized bradykinin. Incubating HK-Lys379 or LK-Lys379 with 40 nM plasmin generated peaks with m/z = 1205.5 and m/z = 1204.8, respectively, representing oxidized Lys-bradykinin. The peak for the reaction with LK-Lys379 and plasmin with m/z = 1189.8 is nonoxidized Lys-bradykinin.
Mass spectrometry. Mass spectrometry (MALDI) analysis of kinins generated by cleavage of WT HK/LK-Met379 and variant HK/LK-Lys379 kininogens (200 nM) by PKa or plasmin. The top 2 elution profiles show results for synthetic bradykinin and kallidin control peptides. For bradykinin, the peak at m/z = 1060.5 is unmodified bradykinin. Additional peaks are likely monosodated and disodated forms of bradykinin. For kallidin, the peak at m/z = 1189.8 is unmodified kallidin. When HK-Met379 and HK-Lys379 are digested with 2 nM kallikrein, peaks with m/z = 1076 were obtained, corresponding to an oxidized form of bradykinin (supplemental Figure 5). Similarly, when HK-Met379 and LK-Met379 were incubated with 40 nM plasmin, peaks with m/z = 1077.2 and m/z = 1076.9, respectively, representing oxidized bradykinin, were obtained. The peak with m/z = 1060.9 for LK-Met379 with plasmin represents unoxidized bradykinin. Incubating HK-Lys379 or LK-Lys379 with 40 nM plasmin generated peaks with m/z = 1205.5 and m/z = 1204.8, respectively, representing oxidized Lys-bradykinin. The peak for the reaction with LK-Lys379 and plasmin with m/z = 1189.8 is nonoxidized Lys-bradykinin.
Consistent with our previous results,27 plasmin released bradykinin from wild-type HK-Met379 and LK-Met379. Again, the major peaks (m/z = 1077.2 and m/z = 1076.9, respectively; Figure 4) are consistent with the addition of a hydroxyl group on Pro383. For LK-Met379, there is also a smaller peak at m/z = 1060.9 matching the bradykinin standard, consistent with partial oxidation at Pro383. In contrast, plasmin cleavage of HK-Lys379 and LK-Lys379 generated major peaks with m/z ratios of 1205.5 and 1204.8, respectively, representing Lys-bradykinin (m/z = 1189) with a hydroxyl group on Pro383. For LK-Lys379 incubated with plasmin there is a second peak (m/z = 1189.8) matching the Lys-bradykinin standard, again consistent with partial oxidation at Pro383. These findings indicate that the Lys379 substitution creates a novel cleavage site for plasmin that is preferred over cleavage after Lys380 in HK and LK, and that the kinin produced as a result is Lys-bradykinin.
Kinin generation in plasma
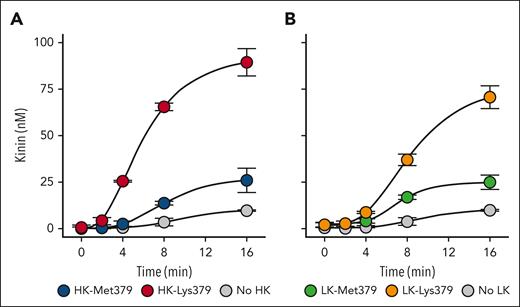
In the original report by Bork et al,29 individuals who were symptomatic were heterozygotes for the Kng1 mutation causing the Lys379 substitutions in kininogens. Their plasmas, therefore, likely contain mixtures of wild-type and variant kininogens. We supplemented normal human plasma with wild-type or variant HK or LK to final concentrations of 100 nM, leaving endogenous plasma HK and LK in excess of the recombinant proteins. The amount of recombinant kininogen was chosen to avoid abnormally high total plasma kininogen concentrations. The plasmas were also supplemented with recombinant Glu-plasminogen.27 Compared with the endogenous Glu-plasminogen in plasma, the recombinant protein is in an open conformation that is activated rapidly by tPA,45,46 facilitating the generation of robust kinin signals in the plasma system. As shown in Figure 5, kinin generation is greater in plasma supplemented with HK-Lys379 (left panel) or LK-Lys379 (right panel) than if supplemented with HK-Met379 or LK-Met379. Signals for HK-Met379 or LK-Met379 were roughly similar. However, given the differences in normal plasma HK and LK concentrations, (640 nM and 2.4 μM, respectively) the results suggest that LK could be the major source of kinins during attacks of HAE.
Kinin generation in normal plasma supplemented with recombinant kininogens. (A) Normal plasma was supplemented with 500 μM captopril, 10 μM KV999272 (kallikrein inhibitor), 600 nM recombinant Glu-plasminogen, 100 nM HK-Met379 or HK-Lys379, or control. (B) As in panel A, except the added kininogen was 100 nM LK-Met379 or LK-Lys379. In both sets of reactions, tPA was added to 125 nM to generate plasmin, and aliquots were taken at indicated time points for measurement of kinin concentration by ELISA. Shown are averages ± 1 standard deviation for 2 runs.
Kinin generation in normal plasma supplemented with recombinant kininogens. (A) Normal plasma was supplemented with 500 μM captopril, 10 μM KV999272 (kallikrein inhibitor), 600 nM recombinant Glu-plasminogen, 100 nM HK-Met379 or HK-Lys379, or control. (B) As in panel A, except the added kininogen was 100 nM LK-Met379 or LK-Lys379. In both sets of reactions, tPA was added to 125 nM to generate plasmin, and aliquots were taken at indicated time points for measurement of kinin concentration by ELISA. Shown are averages ± 1 standard deviation for 2 runs.
Discussion
In this study, replacing Met379 in HK and LK with Lys renders both proteins more susceptible to plasmin-mediated kinin release by creating a novel cleavage site at the N-terminus of the kinin sequence. Plasmin preferentially cleaves the variant kininogens after Lys379 and Arg389 rather than at the sites normally used by PKa for bradykinin release (after Lys380 and Arg389). Consequently, plasmin cleavage of HK-Lys379 and LK-Lys379 generates Lys-bradykinin (kallidin) rather than bradykinin. In plasma in which fibrinolysis is induced by addition of tPA, kinin release was substantially greater in plasma containing HK-Lys379 or LK-Lys379 than in plasma containing wild-type HK-Met379 or LK-Met379, consistent with a role for the Lys379 variants in angioedema. Lys-bradykinin and bradykinin both bind to the B1 and B2 bradykinin receptors and appear to have equipotent effects on cell Ca2+ signaling.13,30,47 Because Lys-bradykinin is readily converted to bradykinin by aminopeptidase B, it is also possible that in carriers of the Lys379 substitution, angioedema may be partly, or largely, driven by bradykinin derived from Lys-bradykinin. To our knowledge, our study is the first to suggest that a form of HAE is due to generation of a kinin other than bradykinin.
Soft tissue swelling in HAE is typically caused by episodic increases in bradykinin generation, distinguishing it from more common histamine-induced swelling during allergic reactions.1,3-5 A defect in regulating PK and FXII activation and PKa and FXIIa activity caused by reduced plasma C1-INH activity accounts for most cases of HAE.1-3,16,17 However, at least 10% of patients with clinical presentations consistent with HAE have normal C1-INH levels (HAEnC1).18-21 Single amino acid substitutions in 6 proteins have been linked to HAEnC1. The first discovered, and most common, are Lys or Arg substitutions for Thr328 in FXII.10,19-22 The introduction of a basic amino acid creates a novel cleavage site for proteases such as plasmin and thrombin, allowing for the removal of the FXII heavy chain region.10,25,26 The resulting truncated FXII is activated by PKa more rapidly than full-length FXII, accelerating KKS activation and overwhelming the capacity of C1-INH to control kinin generation. A Thr144Ser substitution in heparan sulfate 3-O-sulfotransferase 6 may alter glycosylation of proteoglycans on vascular endothelial cell surfaces.48 Bork et al postulated that the mutation causes loss of enzyme function, resulting in changes to key HK binding partners that render the protein more susceptible to cleavage by PKa.48 As with C1-INH deficiency, the proposed mechanisms for HAE in patients with FXII Lys/Arg328 and 3-O-sulfotransferase 6 Thr144Ser involve increased bradykinin production. Two other mutations identified in patients with HAEnC1 may alter vascular endothelium responses to factors that influence vessel permeability. Ala119Ser in the soluble protein angiopoietin-1 causes loss of function that may confer increased endothelial cell sensitivity to permeability-enhancing factors, including bradykinin and vascular endothelial growth factor.49,50 Similarly, an Arg217Ser replacement in the membrane protein myoferlin may lead to increased cell permeability by redistributing vascular endothelial growth factor receptor 2 from the cytoplasm to the cell membrane.51
Interactions between the fibrinolytic system and the KKS were postulated more than 50 years ago. Plasmin converts FXII to FXIIa by proteolysis of the Arg372-Val373 peptide bond.25,27,52,53 Furthermore, FXII truncation by plasmin may contribute to inflammatory disorders including angioedema.11,53,54 There are several reports describing HK cleavage by plasmin, although it is not clear that this results in kinin production in all situations.41-43 Recently, we conducted a head-to-head comparison of the capacities of PKa and plasmin to release bradykinin from HK and LK.27 PKa released bradykinin 50- to 100-fold faster from HK than from LK, whereas plasmin released bradykinin threefold to fourfold faster from LK than from HK. The rate of bradykinin release from HK was at least 50-fold greater for PKa than for plasmin, but the enzymes had similar capacities to release bradykinin from LK.
Several lines of evidence implicate plasmin-catalyzed reactions in angioedema. Observations of patients with congenital bleeding disorders strongly indicate that fibrinolytic activity is particularly robust in the mouth and nasopharynx.55 Plasma bradykinin levels rise after infusions of the fibrinolytic activator tPA during treatment for thrombotic events,56 and in some of these individuals, angioedema develops that is typically confined to the mouth (oral-lingual edema).57,58 Similarly, angiotensin-converting enzyme (ACE) inhibitors can induce oral-lingual angioedema by interfering with kinin degradation.59,60 The predilection for oral-lingual edema with tPA or ACE inhibitor treatment, and in some patients with HAE, may reflect intrinsically higher fibrinolytic activity in the oropharynx. Fibrinolytic inhibitors such as tranexamic acid have been used successfully to treat ACE inhibitor–induced angioedema.61-64 They are also used to treat patients with HAEnC1 and some with HAE due to low C1-INH.65,66 It is not clear why antifibrinolytics seem to be effective in patients with PKa-mediated HAE.
In 2018 Bork et al and Dewald et al described a mutation in the PLG gene encoding plasminogen in some patients with HAEnC1 that causes Lys330 to be replaced with glutamic acid.23,24 The substitution creates a consensus binding site for side chains of basic amino acids such as Lys and Arg. It is notable that 80% of patients with plasminogen-Glu330 experience tongue swelling, and in many, this is the only manifestation of HAE.16-19,23,67 We showed that replacing Lys330 with glutamic acid results in a striking increase in the rate of plasmin-catalyzed bradykinin release from both HK and LK, probably because the plasmin variant binds basic amino acids on kininogens differently than does wild-type plasmin.27 Plasmin-Glu330 catalyzes bradykinin release comparably from HK and LK. The plasma concentration of LK is twofold to fourfold higher than that of HK, and plasma-based experiments support the conclusion that LK may be the major kinin source in carriers of plasminogen-Glu330.27
As with plasminogen-Glu330, the results presented here for kininogens with the Lys379 substitution suggest that angioedema is the result of a mechanism that involves plasmin-mediated cleavage of both HK and LK. However, our work does not rule out the possibility that other proteases cleave the variant kininogens to release kinins. Because trypsin-like enzymes primarily cleave proteins after basic amino acids, introducing Lys379 might have created a cleavage site for proteases that were not identified in our limited survey. Furthermore, LK is primarily a substrate for the tissue kallikrein hK1 (encoded by the KLK1 gene) rather than for PKa (encoded by the KLKB1 gene), and the effects of the Lys379 replacement on LK cleavage by hK1 and other tissue kallikreins are unknown. The data shown here, and prior work with plasminogen-Glu330, support the conclusion that some forms of HAE do not require, and may not involve, PKa and FXIIa. This has implications for treating patients with HAE. Drugs specifically targeting PKa or FXIIa may be less effective for carriers of kininogen Lys379 or plasminogen Glu330 than for patients with other forms of HAE. Agents that block the B2 bradykinin receptor or inhibit fibrinolysis may be more appropriate for patients with angioedema that is primarily plasmin driven. Controlled clinical trials are needed to determine how genetic information in patients with HAEnC1 can be best used to guide selection of treatments.
Acknowledgments
This work was supported by awards HL140025 from the National Institutes of Health, National Heart, Lung, and Blood Institute (D.G.); APP1129592 from the National Health and Medical Research Council Australia (R.H.P.L.); and FL18010019 from the Australian Research Council (R.H.P.L.). Protein and peptide tandem mass spectrometry measurements were supported by the National Institutes of Health, Office of the Director under grant S10 OD025118 (principal investigator: Jon Amster, University of Georgia).
Authorship
Contribution: S.K.D. designed and performed assays for kininogen cleavage and bradykinin generation, performed expression and purification of proteins, and contributed to writing the manuscript; S.K., D.R.P., and E.T.R. designed and performed mass spectrometry analyses; M.-f.S. developed the system for expressing recombinant HK; M.L. studied surface-dependent KKS activation; T.Z.H. performed assays for protease activation and bradykinin generation; R.H.P.L. developed the system for expressing recombinant Glu-plasminogen; E.P.F. helped design experiments involving the kallikrein inhibitor KV999272 and contributed to writing the manuscript; and D.G. oversaw the project and writing of the manuscript.
Conflict-of-interest disclosure: D.G. receives consultation fees from pharmaceutical companies with an interest in inhibition of contact activation and the KKS for therapeutic purposes. E.P.F. is an employee of Kalvista Pharmaceuticals, Inc. The remaining authors declare no competing financial interests.
Correspondence: David Gailani, Pathology, Microbiology, and Immunology, Vanderbilt University Medical Center, Room 4918, The Vanderbilt Clinic, 1301 Medical Center Dr, Nashville, TN 37232; email: dave.gailani@vanderbilt.edu.
References
Author notes
Original data, sequences of cDNA constructs, or cDNA constructs used in this study are available on request from the corresponding author, David Gailani (dave.gailani@vumc.org).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal