Visual Abstract
Hematopoietic stem cells (HSCs) are instrumental for organismal survival because they are responsible for lifelong production of mature blood lineages in homeostasis and response to external stress. To fulfill their function, HSCs rely on reciprocal interactions with specialized tissue microenvironments, termed HSC niches. From embryonic development to advanced aging, HSCs transition through several hematopoietic organs in which they are supported by distinct extrinsic cues. Here, we describe recent discoveries on how HSC niches collectively adapt to ensure robust hematopoietic function during biological aging and after exposure to acute stress. We also discuss the latest strategies leveraging niche-derived signals to revert aging-associated phenotypes and enhance hematopoietic recovery after myeloablation.
Introduction
The spatial microenvironment of hematopoietic stem cells (HSCs) is a multiparametric ecosystem composed of multiple hematopoietic and nonhematopoietic populations, their molecular signals, and extracellular matrix components. From these, a functional subunit called the HSC niche provides lifelong support by promoting HSC quiescence, self-renewal, and niche retention.1 Previous studies used tissue imaging and in vivo cell type–specific ablation approaches to elucidate both the composition and functional roles of putative HSC niches.2 As a result, several bone marrow (BM) populations have been reported to support homeostatic HSCs including endothelial cells (ECs) and mesenchymal stromal cells (MSCs) that promote HSC maintenance and niche retention,3-5 nonmyelinated Schwann cells,6 megakaryocytes (MKs)7,8 and macrophages9 supporting HSC quiescence, and osteolineage cells (OLCs) that have been proposed to regulate HSC numbers.10,11 However, the composition and function of HSC niches exhibit context-dependent adaptation, particularly during aging or response to stress. Here, we review recent findings that have advanced our understanding of the spatial organization and molecular composition of murine and human HSC niches from early development to aging and in response to acute stress.
Spatio-molecular organization of HSC niches throughout life
Embryonic development
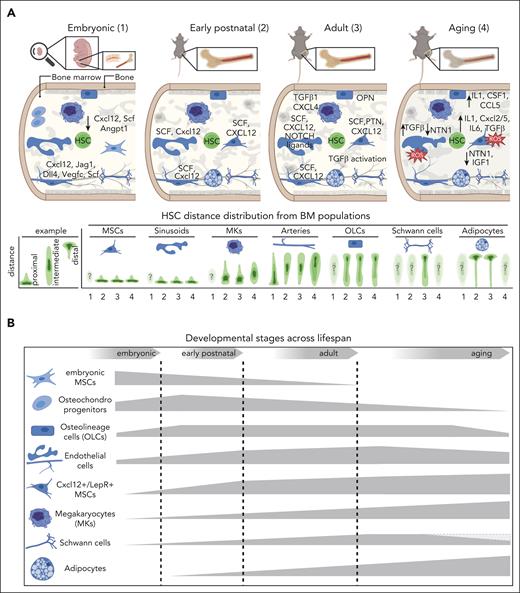
Embryonic HSCs transition through different organs and are supported by distinct niche cues to fulfill their function.12 Murine and human definitive HSCs emerge from arterial-like hemogenic precursors at the aorta-gonad-mesonephros region.12-16 To uncover cell-extrinsic signals that support HSC emergence, Hadland et al compared the transcriptomic profile of murine aorta-gonad-mesonephros–derived ECs with or without hemogenic potential. Cytokine and integrin signaling were enriched in hemogenic ECs, and the respective proteins functionally supported HSCs in vitro (Cxcl12, Notch1/2, Vascular Cell Adhesion Molecule 1 [Vcam1], and Fibronectin).17 In the human system, Crosse et al investigated niche signals enriched in the ventral side of the dorsal aorta. Endothelin-1 (EDN1), a peptide involved in vasoconstriction, was among the most promising ventrally enriched factors due to its colocalization with Runx1+ intra-aortic hematopoietic clusters18 and contribution to the ventral enrichment of tumor necrosis factor α signaling that supports HSC emergence.19 Despite failure to detect EDN1 receptor on HSCs, colony-forming assays and transplantation experiments confirmed EDN1’s role in promoting early HSC development.18 In combination, these studies identified functional cell-extrinsic regulators supporting murine and human HSC emergence.
Next, HSCs migrate to the fetal liver (FL) where they expand in numbers.20 This observation sparked considerable interest in identifying the cell-extrinsic regulators that promoted HSC self-renewal and expansion.21-27 To profile the FL microenvironment in situ, Lu et al used multiplexed error robust fluorescence in situ hybridization and identified 8 major populations including arteriolar and sinusoidal endothelium, hepatocytes, and 5 hematopoietic populations.28 The frequencies and cellular composition of the HSC microenvironment were similar to average FL locations (50% erythroid, 20% hepatocytes, and 5% arteriolar cells), thus providing no evidence for preferential association of HSCs with specific microenvironment(s).28 However, Notch signaling components (Notch3 and delta like ligand 4 [Dll4]) and the arteriolar marker Ephrin-B2 were enriched within HSC microenvironments at the transcriptome28 and protein level.29,30 These results suggest spatially resolved signaling activation, although further studies are required to decipher their functional role on HSC function. Besides ECs, macrophages contribute to FL HSC expansion through cytokine secretion.30 Elegant live-cell imaging in zebrafish embryos demonstrated that macrophages surveil the expanding stem cell pool and engulf HSCs with high levels of reactive oxygen species and higher risk to accumulate DNA damage.31 These data illustrate that the FL microenvironment not only provides supporting signals but also safeguards developmental hematopoiesis by regulating survival of HSC clones with baseline stress levels.
After their FL expansion, HSCs migrate to the fetal spleen and fetal BM (fBM), which becomes the primary hematopoietic organ after birth. HSCs start seeding the fetal spleen by embryonic day 15.5 (E15.5)32; however, its microenvironment has limited capacity to support their self-renewal and instead promotes myeloid differentiation.33 HSC migration to the fBM seems to be independent of the fetal spleen, because fetal spleen removal had no impact on fBM HSC numbers.27,34 Recent studies timed the presence of the first long-term repopulating cell in murine fBM as early as E15.535 or few days later32,36 and 12 weeks after conception in humans.37 Unexpectedly, early repopulating activity does not reside within the “classical” HSC compartment, because E16.5 CD150+CD48–cKit+Sca1+Lineage– fBM HSCs displayed no measurable long-term reconstitution.35 Gene expression and competitive repopulation assays revealed that fBM HSCs are transcriptionally distinct and functionally inferior from their adult counterparts.35 Instead, embryonic FL-derived multipotent progenitors generated in a HSC-independent manner contribute to both embryonic and adult hematopoiesis by near-lifelong blood production.38 These data revised our view of the fBM as a site with limited capacity to support HSCs.
When does the BM acquire its supportive role? The appearance of repopulating cells coincides with tissue calcification and vascularization. Despite their concomitant progression, osteogenesis is uncoupled from angiogenesis. Deletion of Osx-expressing OLCs did not affect vascular development but significantly impaired homing and engraftment of repopulating cells,36 suggesting a functional role of OLCs in fBM colonization. Transcriptomic analyses revealed significant differences in the composition of fetal vs postnatal/adult BM. Contrary to adult BM where they form an extensive network39 and overlap with leptin receptor+ (LepR+) cells,40,41 Cxcl12+ MSCs are absent from fBM (Figure 1). Instead, osteochondrocytes, fibroblasts,35 or, an embryonic MSC population (Col3a1highDecorinhighGsn/Gle3bhigh)42 are the most abundant fBM populations. The absence of Cxcl12+/LepR+ MSCs leads to reduced levels of CXCL12, stem cell factor (SCF), and angiopoietin 1 (ANGPT1) in murine42 and early-stage human fBM,37 which was not compensated by other MSCs42 nor OLCs.36 Expectedly, fBM MSCs displayed inferior capacity to support cocultured HSCs compared with adult MSCs.35 Unlike the MSC compartment, all EC subtypes are present in fBM and share common cytokine profiles with adult BM vasculature (Cxcl12, Scf, and vascular endothelial growth factor C [Vegfc]). However, arteriolar rather than sinusoidal endothelium is the main source of hematopoietic cytokines in fBM (Cxcl12, Jagged-1 [Jag1], and Dll4)37,42 and promotes HSC proliferation via canonical Wnt/β-catenin signaling.42 In summary, murine BM seems to acquire its HSC-supportive role postnatally. Collectively, these studies provide novel insights into the cell-extrinsic signals that support HSCs during their transition through different embryonic stages.
The evolving composition of the BM niche for HSCs from embryonic development to advanced aging. (A) Graphical overview of the cellular and molecular 3-dimensional organization of the HSC niche (upper panel) as well as the HSC distance distribution from putative BM niche populations across life span (lower panel; embryonic: 1; early postnatal: 2; adult: 3; aging: 4). Corresponding factors are in capital letters when protein data are available or in small letters for transcriptomic data. Arrows indicate increase or decrease in expression compared with the adult stage. Topographic maps illustrate the distance distribution of HSCs from putative BM niche populations, with dark green contour areas marking the distance bin where most HSCs are located (lower panel). An example of the expected distance distribution for BM populations being close (proximal) or far away (distal) from HSCs is shown (lower left panel). Transparent topographic maps marked with a question mark represent HSC-niche associations that remain unknown. (B) Graphical representation of dynamic changes in the cellular composition and relative frequency of key BM populations across life span. The thickness of the displayed 2-dimensional shape corresponds to the relative frequency of the respective population per age group. Some populations are frequent at the embryo stage but decrease during adulthood (osteo-chondrocyte progenitors and embryonic MSCs), whereas others increase in adulthood or during aging. A gray shape has been added for Schwann cells to demonstrate contradictory findings. Angpt1, angiopoietin 1; Csf1, colony-stimulating factor 1; Cxcl12, C-X-C motif chemokine ligand 12; CCL5, C-C motif chemokine ligand 5; Dll4, delta like ligand 4; IGF1, insulin-like growth factor 1; Jag1, jagged-1; OPN, osteopontin; PTN, pleiotrophin; ROS, reactive oxygen species; Scf, stem cell factor.
The evolving composition of the BM niche for HSCs from embryonic development to advanced aging. (A) Graphical overview of the cellular and molecular 3-dimensional organization of the HSC niche (upper panel) as well as the HSC distance distribution from putative BM niche populations across life span (lower panel; embryonic: 1; early postnatal: 2; adult: 3; aging: 4). Corresponding factors are in capital letters when protein data are available or in small letters for transcriptomic data. Arrows indicate increase or decrease in expression compared with the adult stage. Topographic maps illustrate the distance distribution of HSCs from putative BM niche populations, with dark green contour areas marking the distance bin where most HSCs are located (lower panel). An example of the expected distance distribution for BM populations being close (proximal) or far away (distal) from HSCs is shown (lower left panel). Transparent topographic maps marked with a question mark represent HSC-niche associations that remain unknown. (B) Graphical representation of dynamic changes in the cellular composition and relative frequency of key BM populations across life span. The thickness of the displayed 2-dimensional shape corresponds to the relative frequency of the respective population per age group. Some populations are frequent at the embryo stage but decrease during adulthood (osteo-chondrocyte progenitors and embryonic MSCs), whereas others increase in adulthood or during aging. A gray shape has been added for Schwann cells to demonstrate contradictory findings. Angpt1, angiopoietin 1; Csf1, colony-stimulating factor 1; Cxcl12, C-X-C motif chemokine ligand 12; CCL5, C-C motif chemokine ligand 5; Dll4, delta like ligand 4; IGF1, insulin-like growth factor 1; Jag1, jagged-1; OPN, osteopontin; PTN, pleiotrophin; ROS, reactive oxygen species; Scf, stem cell factor.
Early postnatal life
Although HSCs expand in the FL before migrating to the BM, elegant lineage tracing experiments have recently challenged the extent to which FL-HSC expansion contributes to the adult HSC pool and lifelong hematopoiesis.43 Instead, FL expansion was reported to favor hematopoietic differentiation, suggesting that the adult BM HSC compartment is independently established postnatally. Indeed, BM HSCs retain active cell-cycling characteristics 4 weeks after birth, before switching to the adult quiescent phenotype.44 Although proposed to be intrinsically determined, this phenotypic HSC switch could be cosupported by the BM niche. In line with this hypothesis, the transcriptional rewiring of BM endothelial and mesenchymal compartments is more pronounced during the first weeks of postnatal life and transition to adulthood than during the 2-year aging period.45,46 Furthermore, sinusoids and Cxcl12+/LepR+ MSCs significantly expand (<1% at perinatal day 4 [P4]) and become among the most prevalent nonhematopoietic BM populations by P1446 (Figure 1B). Concomitantly, most HSCs spatially associate with sinusoids (but only few with arteries, capillaries, or bone) and rely on EC-derived membrane-bound46 and soluble/systemic SCF,47 a role later acquired by Cxcl12+/LepR+ MSCs. Quantitative multiplex 3-dimensional imaging revealed that most adult HSC-niche populations are present in the BM by P21; however, Cxcl12+ MSCs and MKs are less frequent compared with adult BM. Although 5-week-old and adult HSCs shared similar niche signatures, 3-week-old HSCs were enriched in direct contact with CXCL12-Discosoma red fluorescent protein (DsRed) expressing MSCs and away from MKs,48 a cell type reported to promote HSC quiescence. Notably, the reduced HSC-MK association reflected the twofold reduction in MK abundance in preswitch vs adult BM.48 These findings underscore the spatial remodeling of BM tissue as it acquires its HSC-supportive role, as well as its potential contribution to the fetal-to-adult HSC switch.
Adult stage
The adult BM is a highly complex tissue composed of multiple hematopoietic and nonhematopoietic populations including sinusoids, arteries, capillaries, MSCs, OLCs, chondrocytes, adipocytes, and neuronal and smooth muscle cells. HSCs depend on reciprocal interactions with their BM niche to fulfill their role in blood cell production.1,49 In vivo ablation of selected populations and/or factors using reporter mouse models (including those expressing Cre recombinase) has led to the identification of the cellular and molecular BM constituents of the HSC niche (reviewed in detail elsewhere1,2,50). These experiments showed that ECs promote HSC maintenance via the production of SCF5 and CXCL12,51 whereas MSCs support HSC maintenance39 and niche retention via pleiotrophin,52 SCF,5 and CXCL12.3,51 Furthermore, several BM populations regulate HSC quiescence including nonmyelinated Schwann cells (via transforming growth factor β [TGF-β]6), MKs (via TGF-β8 and CXCL47), macrophages (via atypical chemokine receptor 1 [DARC]9), and chondroitin sulfate proteoglycan 4 (NG2) peri-arteriolar cells.53
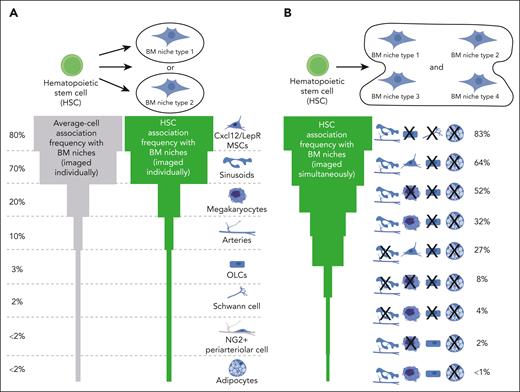
Identifying the anatomical composition of the BM HSC niche in situ is also essential to unravel the cross talk signals supporting HSC function. Surprisingly, homeostatic murine HSCs have been reported in the vicinity of several BM populations but at different frequencies.54 Cxcl12+/LepR+ MSCs3,48,55 and sinusoids48,55,56 are the most frequent cellular components of the HSC microenvironment, because 80% and ∼70% of HSCs locate in their immediate proximity, respectively (Figure 2A). MKs7,8,48 and Nestin-green-fluorescent-protein (GFP) expressing cells53,57 are the next most frequent populations (20% HSCs), followed by arteriolar endothelium (∼10% HSCs).48,53,55 Bone, nonmyelinated Schwann cells, and adipocytes are rarely part of the HSC microenvironment, because most HSCs locate several cell-diameters away (<5% HSCs).6,48,55,56 Notably, the frequency of these associations might marginally vary depending on the utilized HSC identification strategy (Lineage–CD41–CD48–CD150+6-8,53 or α-catulin-GFP+cKit+ HSCs48,55), bone type, and imaged/analyzed volume (single tiles vs volumetric tissue-wide imaging), but have been mostly confirmed by independent studies (reviewed in detail elsewhere54). In human BM, most CD34+ hematopoietic stem and progenitor cells (HSPCs) associate with CD271+ MSCs, ECs, or CD163+ macrophages,58 whereas CD34+CD38– HSPCs are proximal to MKs, sinusoids,59 and, rarely, bone.60 However, due to the low purity of functional HSCs within the imaged populations (1 in 617 CD34+CD38– cells are HSCs61), it is unclear which spatial signatures reflect “true” HSC localization.
Quantification of the spatial associations between homeostatic HSCs and their BM niches. (A) Most imaging studies have quantified HSC localization in relation to individual BM niche population per sample (upper panel). BM niche populations are ranked based on the frequency of HSCs found in their immediate proximity (higher frequency at the top, green shape). To account for the relative abundance of displayed populations, the association frequency of average cells (usually calculated by random dots) is also shown (gray shape). For example, Cxcl12+/LepR+ MSCs are placed at the top, because >80% of both adult HSCs and average cells locate next to them due to the dense MSC network and tissue abundance. (B) Quantification of anatomical HSC association with multiple BM niche populations imaged simultaneously on the same sample is essential to unravel the complexity of the BM HSC niche (10 μm distance). NG2, chondroitin sulfate proteoglycan 4.
Quantification of the spatial associations between homeostatic HSCs and their BM niches. (A) Most imaging studies have quantified HSC localization in relation to individual BM niche population per sample (upper panel). BM niche populations are ranked based on the frequency of HSCs found in their immediate proximity (higher frequency at the top, green shape). To account for the relative abundance of displayed populations, the association frequency of average cells (usually calculated by random dots) is also shown (gray shape). For example, Cxcl12+/LepR+ MSCs are placed at the top, because >80% of both adult HSCs and average cells locate next to them due to the dense MSC network and tissue abundance. (B) Quantification of anatomical HSC association with multiple BM niche populations imaged simultaneously on the same sample is essential to unravel the complexity of the BM HSC niche (10 μm distance). NG2, chondroitin sulfate proteoglycan 4.
It is evident that measuring HSC associations with individual BM populations per sample is insufficient to capture niche complexity. Because several populations functionally support HSCs,1 they may collectively form an “HSC-niche functional unit.” In fact, most of the arteriolar and bone locations harboring HSCs are also rich in sinusoids and MSCs, because hardly any hematopoietic cell can be found further than 30 μm away from peri-sinusoidal locations due to their frequency and tissue distribution.41,48,62 In other words, HSCs previously classified as proximal to infrequent BM populations rarely associate with these cell types exclusively. Multiplex visualization of HSCs with multiple BM components demonstrated their simultaneous physical association with different combinations of BM cells, such as perisinusoidal CCXL12+ MSCs (∼64%) or perisinusoidal MKs in central BM away from bone surfaces and adipocytes (∼32%; Figure 2B).48 Notably, the frequency of murine HSC-niche associations correlated with the abundance of homeostatic BM niches, providing no evidence for active HSC migration toward imaged BM populations or their combinations. These data demonstrate that not all HSCs physically associate with the same BM population(s); however, even higher multiplexing is required to profile these complex spatial interactions.
The fact that fractions of the HSC compartment associate with distinct BM populations may reflect the reported HSC heterogeneity63 and the unique requirements of HSC subsets for cell-extrinsic signals (Figure 3). Along these lines, lineage-biased HSCs have been reported to occupy distinct BM locations with von Willebrand factor+ (VWF+) MK-biased HSCs being close to MKs, whereas VWF– HSCs displaying peri-arteriolar localization.64 MKs are important regulators of HSC quiescence via CXCL4 or TGF-β signals7,8; however, whether quiescent and cycling HSCs occupy distinct BM locations remains controversial.53,55 Of note, dormant long-term label-retaining HSCs that contain most of the transplanted stem cell activity65,66 displayed identical BM localization to sinusoidal, arteriolar, MK niches, and their combinations compared with proliferating HSPCs.48 These data challenged the existence of spatially resolved predefined BM niches that differentially support dormant and activated HSCs. In line with these findings, HSCs and multipotent progenitors displayed similar localization in relation to ECs,56 MSCs,67 and bone surfaces (<5 μm difference), although more systematic analyses are required. Are HSCs migratory within BM tissue and therefore able to receive signals from spatially distinct BM niches in steady state? In vivo live-cell imaging of highly purified homeostatic Mds1GFP/+Flt3Cre HSCs revealed baseline motility compared with downstream progenitors in their native BM microenvironment (<10 μm in 150 minutes).56 These results suggest stable HSC-niche interactions, whereas a tamoxifen-induced labeling system reported heterogeneous motility behaviors (Pdzk1ip1-CreER R26LSL-Tom).68
Possible scenarios for the underlying mechanism governing the spatial and functional interactions between HSCs and their BM niches. Graphical overview of different mechanisms of HSC-niche interactions requiring active HSC migration or niche induction. One proposed mechanism suggests that HSCs actively migrate toward spatially distinct, predefined BM locations and establish stable cell-cell interactions with specific niche populations (blue), whereas other BM niches are distally located and may regulate HSC behavior via secreted factors (gray). Alternatively, HSCs actively migrate and establish transient interactions with defined BM niches in spatially distinct locations or only require exposure to immobilized molecular signals (depending on HSC motility). Considering the transcriptional heterogeneity of BM populations, HSCs may interact with spatially and functionally distinct BM subpopulations, which are currently unknown. In addition, HSCs may induce their own niche by transcriptionally reprogram their neighboring BM populations into functional HSC niches. A combination of these mechanisms or the presence of additional scenarios is also possible (marked by the question mark).
Possible scenarios for the underlying mechanism governing the spatial and functional interactions between HSCs and their BM niches. Graphical overview of different mechanisms of HSC-niche interactions requiring active HSC migration or niche induction. One proposed mechanism suggests that HSCs actively migrate toward spatially distinct, predefined BM locations and establish stable cell-cell interactions with specific niche populations (blue), whereas other BM niches are distally located and may regulate HSC behavior via secreted factors (gray). Alternatively, HSCs actively migrate and establish transient interactions with defined BM niches in spatially distinct locations or only require exposure to immobilized molecular signals (depending on HSC motility). Considering the transcriptional heterogeneity of BM populations, HSCs may interact with spatially and functionally distinct BM subpopulations, which are currently unknown. In addition, HSCs may induce their own niche by transcriptionally reprogram their neighboring BM populations into functional HSC niches. A combination of these mechanisms or the presence of additional scenarios is also possible (marked by the question mark).
Another possibility for the nonuniform HSC distribution is their association with distinct, possibly unknown, subsets of BM cell types, which have been traditionally viewed as homogeneous. Single-cell profiling revealed extensive heterogeneity within BM populations at the transcriptome and protein level.69-72 Recent studies revealed that some of these BM subpopulations are spatially and functionally distinct including the adipo- and osteo-primed Cxcl12+/LepR+ MSCs,71 the osteo-primed osteolectin+ peri-arteriolar LepR+ MSCs that support lymphopoiesis,73 the metaphyseal apelin+ arteriolar ECs supporting HSC maintenance,74 the rare type S ECs regulating epiphyseal osteogenesis and supporting transplanted HSCs,75 as well as the metaphyseal Lin–CD31–Sca1–CD51+PDGFRa+PDGFRb+ OLC and IL7+CXCL12+ MSC subsets supporting lymphopoiesis.67,76 Along the same lines, multiplex imaging identified sinusoids as the primary site for myelopoiesis, with potentially distinct sinusoidal subsets supporting maturation of granulocytes, monocytes, or dendritic progenitors.77 Notably, OLCs display distinct transcriptional profiles depending on their proximity to HSCs.78 Whether HSCs actively migrate toward predefined, spatially and functionally distinct BM niches, or whether specific matrix-immobilized signals suffice, or whether HSCs induce such changes on their neighboring cells requires further investigation (Figure 3). Collectively, these studies highlight the multifaceted heterogeneity of the BM microenvironment at the transcriptional, protein, and spatial level and the need for deeper characterization of functional subpopulations beyond conventional cellular compartmentalization.
Biological aging
Aging induces functional alterations to the hematopoietic compartment and BM microenvironment, which are already evident in middle-aged animals (12 months of age).79,80 Despite the marked increase in the frequency of immunophenotypic HSCs in aged BM tissue, old HSCs display reduced repopulation potential, homing efficiency, and B-/T-lineage output compared with their young counterparts.81-83 Aging-associated effects on hematopoiesis are evolutionary conserved as human HSCs from old individuals also exhibit increased cell frequencies, cycling activity, and myeloid bias.84 Mechanistically, the functional impairment of old HSCs is caused by alterations in their genomic stability, epigenetic profile, and defective DNA repair machinery.80,85,86 Furthermore, data from heterochronic transplantations demonstrated that the aged BM microenvironment also contributes to the aging-associated phenotype of old HSCs. Young HSCs display reduced repopulation efficiency and increased myeloid and impaired lymphoid output upon transplantation in aged mice.87,88 Strikingly, old HSCs retain their myeloid-biased lineage output when transplanted in lethally irradiated young recipients with damaged BM niches,80 but not in sublethally irradiated80 or nonconditioned young BM niches, which seem to restore myeloid skewing.89 Furthermore, young unperturbed BM niches restored the transcriptional but not DNA methylation profile of transplanted aged HSCs. Taken together, these findings indicate that cell-intrinsic and environmental factors collectively contribute to the aging-associated HSC phenotype.
Single-cell profiling and functional assays revealed that several components of the BM microenvironment undergo significant remodeling during aging and instigate aging-related phenotypes on young HSCs (Figure 1). Old MSCs expand, display elevated levels of reactive oxygen species and inflammatory cytokines (C-X-C motif chemokine ligand 2 [Cxcl2] and C-X-C motif chemokine ligand 5 [Cxcl5]) known to impair HSC self-renewal (interleukin-1α/β [IL-1α/β] and IL-6),45,90,91 and induce myeloid skewing on cocultured young HSPCs.92 The differentiation trajectory of old MSCs is imbalanced toward the adipocytic lineage resulting in reduced OLC numbers and aging-related bone loss.88,93 As a result, aged murine94,95 and human96 BM accumulate adipocytes, which are known negative regulators of steady-state hematopoiesis.97 Beyond their decreased frequency, aged OLCs display a proinflammatory signature mediated by colony-stimulating factor 1 (CSF1), receptor activator of nuclear factor kappa B ligand (RANKL), and C-C motif chemokine ligand 5 (CCL5), which further contribute to the myeloid skewing of old HSCs.87,98
Aging also remodels the BM vasculature. Sinusoids become discontinuous and leaky,57,99 whereas the frequency and size of arterioles95,100 and type H vessels93 are decreased. Coculture with old ECs induces aging-associated phenotypes on young HSPCs, but young ECs cannot revert the myeloid bias of old HSCs.99 Sympathetic innervation is also affected by aging, demonstrated by the reduction of tyrosine hydroxylase–expressing nerve fibers in murine BM.95 Recent studies revealed that BM nerves are important mediators of aging phenotypes on the hematopoietic system. Unilateral surgical denervation of femoral and sciatic nerves in young mice induced premature aging via adrenergic receptor β3 signaling (ADRβ3) on both HSCs and BM niches (decreased arteriolar volume and mild expansion of dysfunctional MSCs), respectively.95 Expectedly, the age-related remodeling of the BM microenvironment affects spatial interactions of HSCs with BM niches in both mice and humans. Significantly less old murine HSCs physically associate with arterioles and MKs,57,95 whereas their perisinusoidal localization is maintained (Figure 1A). The reduced association between old HSCs and MKs was recently also observed in human BM,60 suggesting conserved aging-associated phenotypes. However, further studies are required to systematically unravel the exact localization of old HSCs in relation to reported BM niches in mouse models and human samples. Taken together, these data illustrate a functional interconnection between biological aging, BM niche remodeling, and functional defects on HSCs.
Are the functional consequences of organismal aging on the HSC compartment reversible? Although calorie restriction, exercise, or systemic exposure to bloodborne factors failed to rejuvenate old HSCs,80,89 several studies reported that targeting specific niche-derived signals reverted HSC function and aging-associated phenotypes. Because the expression of several BM-derived supportive signals decreases with age, their exogenous administration has been used to rejuvenate old HSCs (Table 1). Ex vivo treatment of old HSCs with thrombin-cleaved osteopontin88 or insulin-like growth factor 179 increased their T- and B-cell lineage output at the expense of myeloid cell production, respectively. Furthermore, infusion of an ADRβ3 agonist (BRL37344) reverted donor chimerism and multilineage reconstitution of old to young HSC levels.95 Along the same lines, administration of netrin-1, a secreted protein mainly produced by ECs and MSCs, partially restored DNA damage response on BM niche cells.101 Although netrin-1 treatment did not alter the frequency of immunophenotypic HSPCs, it led to a fourfold increase of functional HSCs and reverted their engraftment deficiency and their myeloid skewing.101 In addition to supplementation of downregulated niche-derived supportive factors, blocking elevated negative regulators of HSC function has also beneficial effects on reverting aging-associated phenotypes. Administration of TGF-βR1 inhibitor reverted the aging-associated expansion of immunophenotypic HSCs and restored their myeloid lineage output after transplantation.102 Furthermore, counteracting the upregulation of IL-1α/β inflammatory signaling significantly improved lymphopoiesis, restored lymphoid/myeloid differentiation output,103 and improved hematopoietic regeneration in chemotherapy-treated old mice.91 Interestingly, similar results were obtained by modulating the microbiome of aged mice either by 8-week antibiotic treatment103 or fecal microbiota transplants from young mice.104 These results demonstrate reversibility of functional defects of aged HSCs by manipulating niche-derived signals. Further studies are required to develop combinatorial intervention strategies for robust rejuvenation, potentially by simultaneous targeting HSC intrinsic programs and BM niche fitness to efficiently overcome crossrestricting phenotypes.
Hematopoietic rejuvenation strategies targeting niche signals in aging and response to cytotoxic conditioning
| Approach . | Method . | Outcome . | Age, mo . | Reference . |
|---|---|---|---|---|
| Aging: supplementation | ||||
| Insulin-like growth factor 1 | Ex vivo treatment, 100 ng/mL, 7 d | Improved B- and T-cell lineage output | 14 | 79 |
| Recombinant netrin-1 | In vivo, 40 μg/mL, 10 subcutaneous injections in 14 d | Restored DNA damage response on BM niche cells, fourfold increase in functional HSCs, improved engraftment efficiency, and reverted myeloid skewing | 18 | 101 |
| Thrombin-cleaved osteopontin | Ex vivo treatment, 40 ng/mL, 16 h | Improved B- and T-cell lineage output | 20-24 | 88 |
| Fecal microbiota suspension from young mice | In vivo, oral gavage, daily for 4 wk | Improved lymphopoiesis and lymphoid/myeloid differentiation | 20-24 | 104 |
| ADRβ3 agonist (BRL37344) | In vivo delivery with osmotic pump, 12 wk | Reverted donor chimerism and multilineage reconstitution | 20-24 | 95 |
| Young EC | 4-d infusion | Improved engraftment and lymphoid differentiation after whole BM transplantation | 24 | 99 |
| Aging: inhibition | ||||
| TGFbR1 inhibitor (SB-525334) | In vivo, 30 mg/kg, oral gavage, daily for 12 d | Reverted HSC expansion and restored lineage output | 24 | 102 |
| IL-1ra inhibitor (anakinra) | In vivo, 37 ug per mouse, IP injection, daily for 21 d | Improved lymphopoiesis and restored myeloid lineage output | 24 | 103 |
| IL-1ra inhibitor (anakinra) | In vivo, 10 mg/kg, IP injection, daily for 14 d | Improved B- and erythroid-cell recovery after 5-FU | 18-31 | 91 |
| Antibiotic treatment | 8 wk, metronidazole, ampicillin, and vancomycin at 1 mg/mL, neomycin trisulfate 0.5 mg/mL | Improved lymphopoiesis and lymphoid/myeloid differentiation | 24 | 103 |
| Cytotoxic conditioning: supplementation | Stress | |||
| EGF | In vivo, 0.5 mg/kg, daily, 4 doses | Accelerated hematopoietic regeneration, induces cycling, and increased SLAM KSL numbers | 5-FU | 105 |
| IL-1β | In vivo, 0.5 ug per mouse, IP injection, daily | Accelerated regenerative myelopoiesis | 5-FU | 106 |
| G-CSF | In vivo, 5 ug per mouse, IP injection, daily | Time point–dependent acceleration/extension of cGMP formation | 5-FU | 106 |
| Angiopoietin 1 | Adenoviral vectors | Stimulates hematopoiesis, shortened duration of 5-FU induced neutropenia | 5-FU | 107 |
| VEGF-A | Adenoviral vectors | Supports vascular regeneration | 5-FU | 107 |
| VEGF-A | In vivo, 2 μg per mouse per dose, 4 doses IV injection | Supports vascular regeneration and hematopoietic reconstitution, improved overall survival | 9 Gy | 74 |
| 4-MC | In vivo, 10 μg/kg, IP injection, daily for 7 wk | Restoration of HSC frequencies and hematopoietic regeneration | Cisplatin | 108 |
| Isolated and in vitro expanded ILC2 | In vivo, 2 × 105 cells per mouse per dose, 2 doses, IV | Accelerated hematopoietic recovery | 5-FU | 109 |
| Cytotoxic conditioning: inhibition | ||||
| Anti–TGF-β neutralizing antibody (1D11) | In vivo, 10 mg/kg, IP injection, 3 doses | Accelerated regeneration after 5-FU | 5-FU | 110 |
| Anti-NRP1 antibody | In vivo, 10 μg/kg, IP injection, 5 doses | Accelerates vascular remodeling and hematopoietic reconstitution | TBI (5Gy) 5-FU | 111 |
| PAI-1 inhibitor (TM5614) | In vivo, oral gavage, daily for 14 d | Improved hematopoietic regeneration after 5-FU | 5-FU | 112 |
| Approach . | Method . | Outcome . | Age, mo . | Reference . |
|---|---|---|---|---|
| Aging: supplementation | ||||
| Insulin-like growth factor 1 | Ex vivo treatment, 100 ng/mL, 7 d | Improved B- and T-cell lineage output | 14 | 79 |
| Recombinant netrin-1 | In vivo, 40 μg/mL, 10 subcutaneous injections in 14 d | Restored DNA damage response on BM niche cells, fourfold increase in functional HSCs, improved engraftment efficiency, and reverted myeloid skewing | 18 | 101 |
| Thrombin-cleaved osteopontin | Ex vivo treatment, 40 ng/mL, 16 h | Improved B- and T-cell lineage output | 20-24 | 88 |
| Fecal microbiota suspension from young mice | In vivo, oral gavage, daily for 4 wk | Improved lymphopoiesis and lymphoid/myeloid differentiation | 20-24 | 104 |
| ADRβ3 agonist (BRL37344) | In vivo delivery with osmotic pump, 12 wk | Reverted donor chimerism and multilineage reconstitution | 20-24 | 95 |
| Young EC | 4-d infusion | Improved engraftment and lymphoid differentiation after whole BM transplantation | 24 | 99 |
| Aging: inhibition | ||||
| TGFbR1 inhibitor (SB-525334) | In vivo, 30 mg/kg, oral gavage, daily for 12 d | Reverted HSC expansion and restored lineage output | 24 | 102 |
| IL-1ra inhibitor (anakinra) | In vivo, 37 ug per mouse, IP injection, daily for 21 d | Improved lymphopoiesis and restored myeloid lineage output | 24 | 103 |
| IL-1ra inhibitor (anakinra) | In vivo, 10 mg/kg, IP injection, daily for 14 d | Improved B- and erythroid-cell recovery after 5-FU | 18-31 | 91 |
| Antibiotic treatment | 8 wk, metronidazole, ampicillin, and vancomycin at 1 mg/mL, neomycin trisulfate 0.5 mg/mL | Improved lymphopoiesis and lymphoid/myeloid differentiation | 24 | 103 |
| Cytotoxic conditioning: supplementation | Stress | |||
| EGF | In vivo, 0.5 mg/kg, daily, 4 doses | Accelerated hematopoietic regeneration, induces cycling, and increased SLAM KSL numbers | 5-FU | 105 |
| IL-1β | In vivo, 0.5 ug per mouse, IP injection, daily | Accelerated regenerative myelopoiesis | 5-FU | 106 |
| G-CSF | In vivo, 5 ug per mouse, IP injection, daily | Time point–dependent acceleration/extension of cGMP formation | 5-FU | 106 |
| Angiopoietin 1 | Adenoviral vectors | Stimulates hematopoiesis, shortened duration of 5-FU induced neutropenia | 5-FU | 107 |
| VEGF-A | Adenoviral vectors | Supports vascular regeneration | 5-FU | 107 |
| VEGF-A | In vivo, 2 μg per mouse per dose, 4 doses IV injection | Supports vascular regeneration and hematopoietic reconstitution, improved overall survival | 9 Gy | 74 |
| 4-MC | In vivo, 10 μg/kg, IP injection, daily for 7 wk | Restoration of HSC frequencies and hematopoietic regeneration | Cisplatin | 108 |
| Isolated and in vitro expanded ILC2 | In vivo, 2 × 105 cells per mouse per dose, 2 doses, IV | Accelerated hematopoietic recovery | 5-FU | 109 |
| Cytotoxic conditioning: inhibition | ||||
| Anti–TGF-β neutralizing antibody (1D11) | In vivo, 10 mg/kg, IP injection, 3 doses | Accelerated regeneration after 5-FU | 5-FU | 110 |
| Anti-NRP1 antibody | In vivo, 10 μg/kg, IP injection, 5 doses | Accelerates vascular remodeling and hematopoietic reconstitution | TBI (5Gy) 5-FU | 111 |
| PAI-1 inhibitor (TM5614) | In vivo, oral gavage, daily for 14 d | Improved hematopoietic regeneration after 5-FU | 5-FU | 112 |
4-MC, 4-methylcatechol; cGMP, compact granulocyte/macrophage progenitor cluster; EGF, epidermal growth factor; G-CSF, granulocyte colony stimulating factor 3; IL-1ra, interleukin 1 receptor antagonist; KSL, cKit+Sca1+Lin−; SLAM, signaling lymphocytic activation molecule (CD150+CD48−); TGFbR1, transforming growth factor β receptor 1.
BM tissue remodeling and hematopoietic recovery after acute stress
Before the onset of aging-associated phenotypes, systemic exposure to acute stress elicits damage responses on adult BM and hematopoietic113 tissues that are reminiscent of aging. BM sinusoids and MSCs from adult mice exposed to infection-mimicking agents exhibited significant overlap with those from untreated old mice, particularly in inflammatory and stress-associated transcriptional programs.45 In the context of nonlethal dose of medically relevant cytotoxic conditioning (150 mg/kg 5-fluorouracil [5-FU] or <6Gy total body irradiation [TBI]), HSC-driven hematopoietic recovery is regulated by cell-intrinsic programs (reviewed in detail elsewhere114) and cell-extrinsic signals. Below, we focus on how BM niches adapt and collectively interact to support BM recovery in response to cytotoxic conditioning.
Supportive BM niches in homeostasis and response to cytotoxic conditioning
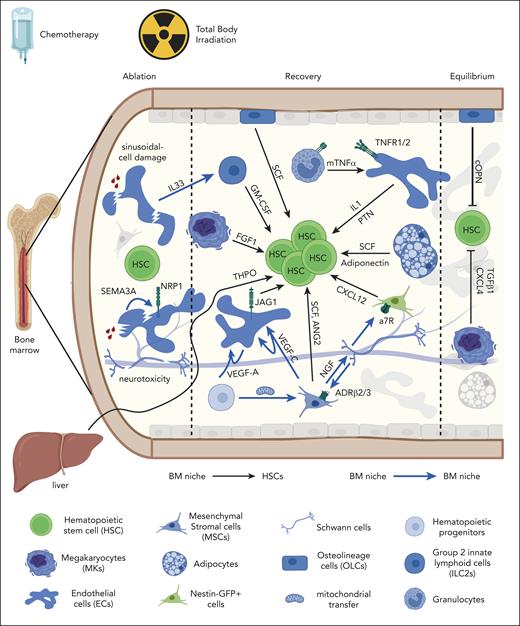
Although the role of several populations is context dependent, BM ECs are critical for both steady-state and emergency hematopoiesis. Ubiquitous vascular endothelial growth factor receptor 2 (VEGFR2) deletion required for sinusoidal regeneration115 or EC-specific Jag1 deletion116 compromised hematopoietic recovery in irradiated mice. Conversely, genetically induced rescue of EC survival significantly improved HSC numbers in chemotherapy-treated mice lacking the preapoptotic factor Bax.105 Mechanistically, EC recovery relies on vascular endothelial growth factor A (VEGF-A) from hematopoietic progenitors74,115 and ECs,117 tumor necrosis factor α from granulocytes,118 as well as VEGF-C from ECs and MSCs119 (Figure 4). The functional relevance of synergistic interactions between ECs and hematopoietic cells is further highlighted by the fact that blood cell ablation phenocopies stress-induced vascular defects.74 During the ablation phase, ECs undergo p53/CDK5-mediated apoptosis by upregulating SEMA3A (semaphorin 3A) and its receptor neuropilin 1 (NRP1).111 Systemic NRP1 inhibition or EC-specific NRP1/SEMA3A deletion accelerated vascular remodeling and hematopoietic reconstitution after cytotoxic conditioning (Table 1) but did not prevent early EC damage.111 During the recovery phase, upregulation of VEGF-A, angiopoietin 1/Tie2, and pleiotrophin signaling facilitates both EC and blood cell regeneration.52,107 Notably, cytotoxic conditioning differentially affects EC populations causing dose-dependent alterations on sinusoidal vessels,115 whereas arteriolar endothelium is mostly preserved.74 After irradiation injury, a rare apelin+ EC subpopulation expands and functionally contributes to murine EC and hematopoietic recovery.74 Similar emergency mechanisms exist in humans as a rare CD105+CD31+ EC subpopulation significantly expands after chemotherapy and promotes hematopoietic and EC regeneration via IL-33.120 Collectively, these data highlight that EC regeneration is a prerequisite for hematopoietic recovery after myeloablation.
Dynamic adaptation of BM niches supporting hematopoietic recovery after cytotoxic conditioning (chemotherapy or TBI). Cellular and molecular composition of BM niche signals supporting HSCs (black arrows) or other BM niches (blue arrows) during ablation, recovery, and return-to-equilibrium phases. Effects on BM niches are mentioned (sinusoidal-cell damage and neurotoxicity). BM niche populations are mentioned on the legend (lower panel). a7R, alpha-7 nicotinic receptor; cOPN, cleaved osteopontin; FGF1, fibroblast growth factor 1; GM-CSF, granulocyte-macrophage colony-stimulating factor; Jag1, jagged-1; mTNF-α, membrane bound tumor necrosis factor α; NGF, nerve growth factor; PTN, pleiotrophin; SCF, stem cell factor; THPO, thrombopoietin; TNFR, TNF receptor.
Dynamic adaptation of BM niches supporting hematopoietic recovery after cytotoxic conditioning (chemotherapy or TBI). Cellular and molecular composition of BM niche signals supporting HSCs (black arrows) or other BM niches (blue arrows) during ablation, recovery, and return-to-equilibrium phases. Effects on BM niches are mentioned (sinusoidal-cell damage and neurotoxicity). BM niche populations are mentioned on the legend (lower panel). a7R, alpha-7 nicotinic receptor; cOPN, cleaved osteopontin; FGF1, fibroblast growth factor 1; GM-CSF, granulocyte-macrophage colony-stimulating factor; Jag1, jagged-1; mTNF-α, membrane bound tumor necrosis factor α; NGF, nerve growth factor; PTN, pleiotrophin; SCF, stem cell factor; THPO, thrombopoietin; TNFR, TNF receptor.
MSCs are critical for steady-state hematopoiesis4,5; however, their role in blood cell recovery has been less understood until recently. Human MSCs are considered radio resistant,121 whereas murine BM MSCs decreased after TBI.72 TBI induces metabolic dysfunction on PDGFRa+Sca1– MSCs, which is, at least partially, repaired by mitochondrial transfer from neighboring HSPCs.122 LepR+ MSCs produce VEGF-C and restore sinusoidal ECs and hematopoiesis after TBI.119 Furthermore, MSCs support sympathetic nerve recovery and hematopoietic regeneration via secretion of nerve growth factor123 counteracting restricting parasympathetic signals.124 In addition to their homeostatic role in regulating HSC quiescence6 and circadian mobilization,125 sympathetic BM nerves also support hematopoietic recovery. Repetitive cycles of neurotoxic chemotherapy (cisplatin or vincristine) caused sympathetic nerve damage and impaired hematopoietic recovery in mice, similar to iatrogenic neuropathy observed in patients who survive cancer.126 Conversely, HSC frequencies and hematopoietic regeneration were restored after genetic or chemically mediated neuroprotection via conditional Trp53 deletion in sympathetic neurons or 4-methylcatechol administration in vivo.108 To exert their supportive role, BM nerves activate ADRβ2/β3 on LepR+ MSCs123 or a7 nicotinic receptor signaling on Nestin-GFP cells127 to increase the expression of key hematopoietic cytokines (SCF, VEGF, angiopoietin 2 [ANG2], and CXCL12). In summary, these findings illustrate that ECs, MSCs, and neurons retain their supportive homeostatic roles and collectively promote BM tissue recovery and hematopoietic regeneration after cytotoxic conditioning.
Context-dependent roles of BM niches after cytotoxic conditioning
Several BM niche populations adapt in response to cytotoxic stress and regulate HSC behavior in a distinct manner from their steady-state role. In homeostasis, MKs promote HSC quiescence via TGF-β1/SMAD8 and CXCL4.7 However, after 5-FU administration, MKs transiently support HSC proliferation via fibroblast growth factor 1 signaling,8 before reinstating HSC quiescence.110,106 This stage-resolved regulation is pivotal for robust hematopoietic regeneration because MK ablation compromises HSC function and emergency myelopoiesis.106 Conversely, transient TGF-β inhibition prolongs HSC cycling and enhances hematopoietic regeneration.110 In addition to MKs, BM-resident group 2 innate lymphoid cells (ILC2) transiently support hematopoietic proliferation after 5-FU. During the ablation phase, dying B-cell progenitors,109 and sinusoids120 release IL-33, which activates ILC2s. Activated ILC2s produce granulocyte-macrophage colony-stimulating factor that supports hematopoietic recovery,109 despite being dispensable for steady-state hematopoiesis.128 Similarly, lymphatic vessels expand in a VEGFR3-/IL-6–dependent manner and support hematopoietic regeneration after stress, although not required for homeostatic HSCs.129
Despite being the first population reported to regulate HSCs,1 OLCs do not seem to directly support homeostatic HSCs.5,49 OLCs are resistant to cytotoxic injury,115 although later studies confirmed radio-resistance of osteoprogenitors rather than mature osteoblasts.72 OLCs were thought to support hematopoietic recovery via thrombopoietin/thrombopoietin receptor (MPL) signaling130; however, recent data identified hepatocytes as the main functional thrombopoietin source after myeloablation.131 Nevertheless, a radio-resistant OLC subpopulation (CD73+ nerve growth factor receptorhigh [NGFRhigh] Runx2+) has emerged as a top hematopoietic cytokine producer that supports myeloid cell recovery after irradiation and hematopoietic transplantation.72 Furthermore, N-Cadherin+ OLCs are reported as functional sources of SCF because their conditional deletion in chemotherapy-treated mice led to reduced HSPC numbers but not BM cellularity.132 Nevertheless, further studies are required to determine the magnitude of OLC's contribution to hematopoietic recovery after myeloablation.
Finally, some BM populations switch from suppressive to supportive roles. Adipocytes are negative regulators of steady-state hematopoiesis, with their abundance inversely correlating with HSC frequencies.97,112 In contrast, they transiently expand after myeloablation and promote hematopoietic recovery through SCF and adiponectin production,133 before transdifferentiating toward MSCs.134 It is possible that adipocytes act as transient mediators of hematopoietic recovery until (peri)vascular BM niches are restored. These data highlight the plasticity of several BM populations and the ability to adapt to extrinsic stress and fulfill hematopoietic demands.
Stress-induced remodeling of spatial HSC localization in relation to putative BM niches
How does acute stress affect the interaction between HSCs and their BM niches? Despite growing evidence for the role of BM niche populations in supporting hematopoietic regeneration, little is known on how myeloablation affects HSC localization. Recent studies reported that expression of CD49b separates chemotherapy-sensitive from chemotherapy-resistant HSCs (CD49b–CD48–CD34–Flk2–cKit+Sca1+Lineage–). Three days after 5-FU, chemotherapy-resistant HSCs are reported to associate with endosteal N-Cadherin+ OLCs, whereas no enrichment was observed toward vessels nor MKs.132 Interestingly, whether HSC localization is re-established after chemotherapy administration seems to be age dependent. Although young HSCs seem to re-establish spatial associations with sinusoids 30 days after 5-FU administration, aged HSCs are reported to migrate from sinusoidal toward arteriolar locations in 18-month-old mice.57
Taken together, these data provide evidence for the dynamic role of BM niches in differentially regulating HSC fate decisions in homeostasis and response to stress, while highlighting the need for deeper longitudinal analysis of HSC localization.
Conclusions and future directions
The application of single-cell and spatial technologies has considerably improved our understanding of BM complexity and its remarkable ability to adapt to organismal demands and extrinsic challenges. It becomes increasingly evident that HSC function is constantly regulated by a complex integrated framework of cell-intrinsic programs and cell-extrinsic signals from multicellular BM niches. Leveraging our mechanistic understanding of the underlying programs toward developing potent intervention strategies could improve the clinical outcome of several conditions. For example, developing combinatorial approaches to simultaneously revert cell-intrinsic vulnerabilities of aged HSCs and rejuvenate their BM niche(s) could significantly enhance regeneration of aged BM tissue. Such findings have the potential to revolutionize treatment of diseases with unfavorable outcome in older patients, such as hematologic malignancies, in which robust hematopoietic function requires both efficient elimination of malignant clones and lifelong healthy hematopoiesis. To achieve clinical translation, thorough preclinical analyses of human HSCs in large cohorts of BM biopsies are essential. The low purity of currently used markers for in situ identification of human HSCs and the need for large-tissue volumetric imaging to unravel 3-dimensional BM niches remain major challenges. To address those, establishing more elegant marker-combination strategies and imaging modalities to perform volumetric imaging of human BM samples is pivotal. Finally, the development of human BM organoids135 provides a unique platform to functionally assess intervention strategies aiming to enhance human hematopoiesis in the context of aging or myeloablation in a controlled system.
Acknowledgments
The authors apologize to all researchers whose work could not be cited because of space limitations. The authors thank all members of the Kokkaliaris lab for their feedback on the text and the figures. All figures were created with BioRender.com.
K.D.K. is funded by the Mildred Scheel Career Center Frankfurt (Deutsche Krebshilfe), the Else Kröner-Fresenius-Stiftung, and the European Hematology Association. J.H. is supported by the Else Kröner-Fresenius-Stiftung.
Authorship
Contribution: J.H. and K.D.K. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Konstantinos D. Kokkaliaris, University Hospital and Goethe University Frankfurt, Theodor-Stern-Kai 7, 60596 Frankfurt am Main, Germany; email: kokkaliaris@med.uni-frankfurt.de.
References
Author notes
Data are available on request from the corresponding author, Konstantinos D. Kokkaliaris (kokkaliaris@med.uni-frankfurt.de).



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal