In this issue of Blood, Dei Zotti et al1 investigated immune checkpoint inhibitor (ICPi)-induced autoimmune hemolytic anemia (AIHA). A subset of CD4+ T cells, characterized as CD39+CD73−FOXP3−CD25−CD4+ (CD39SP) and exhibiting effector/helper T-cell properties, was detectable in ICPi-induced AIHA. Since CD39 functions as an ectonucleotidase breaking down adenosine triphosphate (ATP), enhancing ATPase activity by administering apyrase (an enzyme that degrades ATP) reduced the CD39SP subset and alleviated AIHA progression in the mouse model.
ICPis have revolutionized cancer treatment by enhancing the immune system’s ability to target tumors more effectively.2 However, this advancement comes with the risk of autoimmune disorders, such as AIHA. Although the incidence is relatively low, with approximately 80 cases reported in the Food and Drug Administration database and other literature up to 2019,3 all instances are classified as serious. Currently, treatment options for this complication are limited to steroids and supportive care, such as transfusions.3
The mechanism by which ICPis cause AIHA remains largely unknown. It is speculated that ICPis cause AIHA by enhancing or redirecting immune surveillance, in contrast to other drug-induced AIHA due to immunoallergy or drug-independent autoantibodies because of molecular mimicry.4 Dei Zotti et al used a rodent model where mice expressing a red blood cell (RBC)-specific triple fusion protein composed of hen egg lysozyme, ovalbumin, and human blood group molecule Duffy (HOD)5 were bred with OTII T-cell-receptor transgenic mice.6 Despite this, autoreactive CD4+ T cells in these mice are nonfunctional and subject to peripheral tolerance until tolerance fails with aging, leading to AIHA.6 In this study, instead of using aged mice, ICPi cocktail containing blocking antibodies against PD-1, Lag-3, CTLA-4, and IL-10R was injected into young HOD+OTII+ mice, resulting in AIHA, characterized by high levels of RBC autoantibodies and anemia.1 To a lesser extent, ICPi treatment also accelerated autoimmunity in New Zealand Black (NZB) mice, a well-established model for lupus, and induced autoantibodies in healthy B6 mice.1
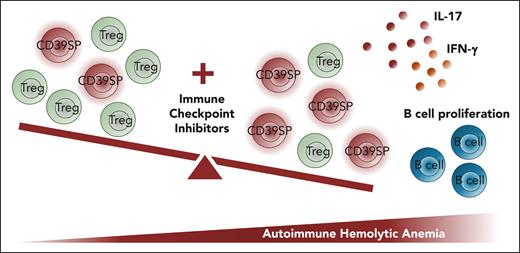
In the ICPi-treated mice, there is an imbalance between regulatory and proinflammatory T cells (see figure).1 A unique T-cell subset, CD39SP, was detected in ICPi-treated young HOD+OTII+ mice, NZB mice, and healthy B6 mice.1 It is notable that using multiple ICPis may have compounding and preferential effects on certain types of T cells.7,8 However, in clinical settings, most patients will receive only 1 single type or at most 2 types of ICPis.2 Therefore, the physiological relevance of the effects caused by the combined ICPis, leading to the discovery of CD39SP, needs further validated in human samples. Indeed, Dei Zotti et al reported that CD39SP T cells were present in 9 patients with AIHA,1 primarily due to lymphoproliferative disorders including 4 with chronic lymphoblastic leukemia, one with follicular lymphoma, and one with angioimmunoblastic T-cell lymphoma. Notably, these patients did not receive ICPi treatment, and only 2 subjects showed a clearly increased percentage of CD39SP cells.
In aged HOD+OTII+ mice without ICPi treatment, CD39SP was increased in mice with detectable levels of autoantibodies.1 This is interesting because it shows that CD39SP cells may have broader implication beyond AIHA secondary to ICPi treatment.
The authors demonstrated that the CD39SP cells were metabolically active, produced proinflammatory cytokines upon restimulation, and stimulated B-cell proliferation (see figure). CD39, a rate-limiting ectonucleotidase, hydrolyses extracellular ATP to adenosine, creating an immunosuppressive environment. CD39 is primarily described as a marker for FOXP3+ regulatory T cells (Tregs),9 but it is also reported to be expressed on FOXP3−CD4+ effector T cells.10 The mechanisms by which CD39SP cells are stimulated and expanded in these AIHA models warrant further investigation.
The study also demonstrated that injection of apyrase, an enzyme that degrades ATP, alleviated AIHA with reduced autoantibodies, improved anemia, and increased probability of survival.1 This has significant implications for the future development of potential treatment options when CD39SP cells are increased and pathogenic during disease progression.
In conclusion, this study advances the understanding of underlying mechanisms of ICPi-induced AIHA, suggests that CD39SP may be an early predicator of potential ICPi-induced AIHA, and indicates that apyrase may be an attractive treatment where CD39SP is pathogenic. A critical next step is to validate the broader presence of increased CD39SP in idiopathic AIHA and other types of AIHA secondary to different conditions.
Combined immune checkpoint inhibitors disrupt T-cell tolerance, leading to the expansion of CD39SP T cells and the onset of autoimmune hemolytic anemia (AIHA). Combined ICPis (blocking antibodies against PD-1, Lag-3, CTLA-4, and IL-10R) promote the expansion of CD39SP, causing an imbalance between Treg and effector T cells. CD39SP cells release proinflammatory cytokines interleukin-17 (IL-17) and interferon-γ (IFN-γ), stimulate B-cell proliferation, and promote the development of AIHA.1
Combined immune checkpoint inhibitors disrupt T-cell tolerance, leading to the expansion of CD39SP T cells and the onset of autoimmune hemolytic anemia (AIHA). Combined ICPis (blocking antibodies against PD-1, Lag-3, CTLA-4, and IL-10R) promote the expansion of CD39SP, causing an imbalance between Treg and effector T cells. CD39SP cells release proinflammatory cytokines interleukin-17 (IL-17) and interferon-γ (IFN-γ), stimulate B-cell proliferation, and promote the development of AIHA.1
Conflict-of-interest disclosure: J.Y.C. reports no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal