Key Points
Patients with Wiskott-Aldrich syndrome can develop inflammatory manifestations poorly controlled by corticosteroid therapy.
Treatment with anti–IL-1 agents resulted in dramatic responses, suggesting an autoinflammatory pathogenesis.
Visual Abstract
Up to 70% of patients with Wiskott-Aldrich syndrome (WAS) develop autoimmune and inflammatory manifestations. Dysregulation of interleukin 1 (IL-1) may be involved in their pathogenesis, yet there is little evidence on treatment with anti–IL-1 agents in these patients. We conducted a multicenter retrospective analysis of 9 patients with WAS treated with anti–IL-1 agents (anakinra or canakinumab). All patients had prominent inflammatory manifestations, including systemic, cutaneous, articular, and intestinal symptoms; 3 patients presented with a severe systemic inflammatory syndrome since the first months of life. Corticosteroid therapy was associated with partial or no response, whereas treatment with anakinra or canakinumab resulted in prompt, often dramatic, responses in all patients, allowing bridging to gene therapy (4 patients) or hematopoietic stem cell transplantation (HSCT; 5 patients). Treatment was overall well tolerated. Low donor myeloid chimerism developed in 4 patients after HSCT and was associated with the appearance or the recurrence of inflammatory manifestations. A second HSCT was performed in 2 patients, achieving full-donor chimerism and resolution of inflammatory manifestation, whereas the other 2 patients were treated with prolonged therapy with anti–IL-1 agents. Our experience demonstrates that some inflammatory manifestations of WAS are dependent on IL-1 and respond well to its pharmacologic blockade.
Introduction
Wiskott-Aldrich syndrome (WAS; Online Mendelian Inheritance in Man number 301000) is a rare primary immunodeficiency disorder caused by mutations in the WAS gene characterized by immunodeficiency, thrombocytopenia, and eczema.1 Up to 70% of patients develop autoimmune and inflammatory disorders, including autoimmune cytopenias, arthritis, vasculitis, and inflammatory bowel disease.2,3 Furthermore, autoimmune and inflammatory disorders complicate allogeneic hematopoietic stem cell transplantation (HSCT) in up to 20% of subjects, particularly when chimerism is low.4,5 The pathogenesis of autoimmunity in WAS has been shown to be related to defective T- and B-cell function.6-8 However, there is increasing evidence for abnormal inflammasome activation and release of proinflammatory cytokines, especially interleukin 1 (IL-1) and IL-18, in WAS.9,10 Therapies aimed at IL-1 blockade may, therefore, represent an effective strategy, yet there is limited clinical information available. Here, we describe a cohort of patients with WAS treated with anti–IL-1 therapies.
Study design
A retrospective review of clinical records of patients with WAS treated with anti–IL-1 agents (anakinra or canakinumab) was performed at 7 pediatric immunology centers. Therapy with anti–IL-1 agents was administered off label after institutional ethical approval, and parents/guardians provided informed consent. All patients were enrolled in locally approved research protocols. The present study has been approved by the Institute for Maternal and Child Health IRCCS “Burlo Garofolo” Institutional Review Board (RC 30/22). Written consent for publication in accordance with the Declaration of Helsinki was obtained from parents or guardians.
Results and discussion
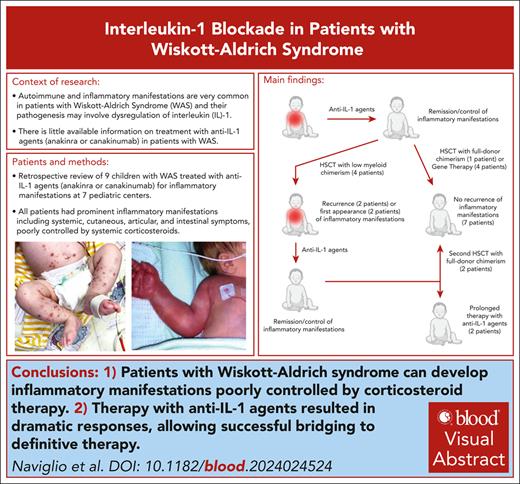
Nine patients were identified; for 4 patients (patients 5, 6, 8, and 9), partial clinical information had been reported elsewhere.10-12 Patients’ clinical characteristics and details of treatment with anti-IL-1 agents are summarized in Table 1 (full clinical and therapeutic history in supplemental Data, available on the Blood website). All patients had prominent inflammatory manifestations (IMs). WAS clinical score, according to Zhu et al,13 was 5A in all patients. Most commonly reported IMs included systemic, cutaneous, articular, and intestinal signs/symptoms. Inflammatory cutaneous involvement, eczema aside, was present in 8 of 9 patients and included cutaneous vasculitis (n = 5), pyoderma gangrenosum (n = 2), recurrent/migrating erysipelas-like lesions (n = 2), and sterile abscess, pustular psoriasis, and panniculitis (n = 1 each) (Figure 1, A-F). Inflammatory joint involvement and inflammatory bowel disease were present in 4 patients each. Three patients (patients 1, 5, and 8) presented with a severe systemic inflammatory syndrome appearing in the first months of life, characterized by prominent fever and systemic symptoms associated with cutaneous inflammatory involvement (rash, vasculitis, and erysipelas-like lesions), failure to thrive, and marked increase of blood inflammatory markers. In the other patients, IMs were not associated with persistent fever and tended to have a more chronic course.
Clinical features and details of anti-IL-1 therapy in patients
| Pt no. . | Genetic mutation . | Age at presentation of IMs, y . | Prominent fever or systemic symptoms . | Skin involvement (beside eczema) . | Arthritis . | IBD . | Response to CS . | Anti-IL1 drug (age at first use, y) . | Drug dose . | Response to anti-IL1 therapy . | Effect of therapy suspension . | Adverse effects of therapy . | Therapy duration, y . | Reason for discontinuation . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | c.276_279dup | 0.2 | Yes | Vasculitis, erythematous papules, scalp blisters | Hands/feet | No | Partial | Anakinra (0.25) | 3 mg/kg/d | Complete | Not tried | No | 0.25 | DT |
| 2 | c.397G>A | 4 (1 y after 1st HSCT) | No | Vasculitis-like lesions of left foot/hand | Ankle, hands | No | Complete | Colchicine (5) | 50 mcg/kg/d | Partial | Not tried | No | 1 | Loss of efficacy |
| Anakinra (6) | 1.5 mg/kg/d | Complete | Symptoms recurrence | No | 2 | DT | ||||||||
| 3 | del Xp11.23 | 5.5 (5 y after HSCT) | No | Erysipelas-like inflammation of lower limbs | Knees, ankles | No | Partial | Anakinra (14) | 3 mg/kg/d | Complete | Not tried | Injection site pain | 0.1 | Adverse effects |
| Canakinumab (14.2) | 2 mg/kg/4 wk | Complete | Not tried | No | 1 | Ongoing | ||||||||
| 4 | c.712_713delAT | 4 | No | Pyoderma gangrenosum, sterile abscesses | No | No | Partial | Canakinumab (7.5) | 4 mg/kg/4 wk | Complete | Symptoms recurrence | No | 4 | DT |
| 5 | c.1225G>T | At birth | Yes | Necrotizing vasculitis, rash, panniculitis, pustular psoriasis | No | Yes | Partial | Anakinra (0.1) | 5 mg/kg/d | Complete | Symptoms recurrence | Lipohypertrophy, injection anxiety | 8 | Adverse effects |
| Colchicine (3) | 50 mcg/kg/d | ND (given with anakinra) | Yes | No | 4 | Not effective | ||||||||
| Canakinumab (8) | 3.5-5.6 mg/kg/4 wk | Complete | Symptoms recurrence | No | 1 | Ongoing | ||||||||
| 6 | c.97C>T | At birth | No | No | No | Yes | Partial | Anakinra (0.58) | 3-4 mg/kg/d | Partial | Not tried | No | 0.66 | DT |
| 7 | c.160_165del | 1.75 | No | Cutaneous vasculitis and edema | Ankle | Yes | Partial | Anakinra (1.75) | 2 mg/kg/d | Complete | Symptoms recurrence | No | 0.3 | DT |
| 8 | c.1384_1385delAG | 0.2 | Yes | Migrating erysipelas-like lesions; vasculitis | No | No | No | Anakinra (0.3) | 5 mg/kg/d | Complete | Symptoms recurrence | Hypereosinophilia | 0.75 | DT |
| 9 | inv(X)g.5721-11840 | 1.5 | No | Pyoderma gangrenosum | No | Yes | Partial | Anakinra (11.8) | 3 mg/kg/d | Complete | Symptoms recurrence | No | 0.6 | DT |
| Pt no. . | Genetic mutation . | Age at presentation of IMs, y . | Prominent fever or systemic symptoms . | Skin involvement (beside eczema) . | Arthritis . | IBD . | Response to CS . | Anti-IL1 drug (age at first use, y) . | Drug dose . | Response to anti-IL1 therapy . | Effect of therapy suspension . | Adverse effects of therapy . | Therapy duration, y . | Reason for discontinuation . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | c.276_279dup | 0.2 | Yes | Vasculitis, erythematous papules, scalp blisters | Hands/feet | No | Partial | Anakinra (0.25) | 3 mg/kg/d | Complete | Not tried | No | 0.25 | DT |
| 2 | c.397G>A | 4 (1 y after 1st HSCT) | No | Vasculitis-like lesions of left foot/hand | Ankle, hands | No | Complete | Colchicine (5) | 50 mcg/kg/d | Partial | Not tried | No | 1 | Loss of efficacy |
| Anakinra (6) | 1.5 mg/kg/d | Complete | Symptoms recurrence | No | 2 | DT | ||||||||
| 3 | del Xp11.23 | 5.5 (5 y after HSCT) | No | Erysipelas-like inflammation of lower limbs | Knees, ankles | No | Partial | Anakinra (14) | 3 mg/kg/d | Complete | Not tried | Injection site pain | 0.1 | Adverse effects |
| Canakinumab (14.2) | 2 mg/kg/4 wk | Complete | Not tried | No | 1 | Ongoing | ||||||||
| 4 | c.712_713delAT | 4 | No | Pyoderma gangrenosum, sterile abscesses | No | No | Partial | Canakinumab (7.5) | 4 mg/kg/4 wk | Complete | Symptoms recurrence | No | 4 | DT |
| 5 | c.1225G>T | At birth | Yes | Necrotizing vasculitis, rash, panniculitis, pustular psoriasis | No | Yes | Partial | Anakinra (0.1) | 5 mg/kg/d | Complete | Symptoms recurrence | Lipohypertrophy, injection anxiety | 8 | Adverse effects |
| Colchicine (3) | 50 mcg/kg/d | ND (given with anakinra) | Yes | No | 4 | Not effective | ||||||||
| Canakinumab (8) | 3.5-5.6 mg/kg/4 wk | Complete | Symptoms recurrence | No | 1 | Ongoing | ||||||||
| 6 | c.97C>T | At birth | No | No | No | Yes | Partial | Anakinra (0.58) | 3-4 mg/kg/d | Partial | Not tried | No | 0.66 | DT |
| 7 | c.160_165del | 1.75 | No | Cutaneous vasculitis and edema | Ankle | Yes | Partial | Anakinra (1.75) | 2 mg/kg/d | Complete | Symptoms recurrence | No | 0.3 | DT |
| 8 | c.1384_1385delAG | 0.2 | Yes | Migrating erysipelas-like lesions; vasculitis | No | No | No | Anakinra (0.3) | 5 mg/kg/d | Complete | Symptoms recurrence | Hypereosinophilia | 0.75 | DT |
| 9 | inv(X)g.5721-11840 | 1.5 | No | Pyoderma gangrenosum | No | Yes | Partial | Anakinra (11.8) | 3 mg/kg/d | Complete | Symptoms recurrence | No | 0.6 | DT |
Abbreviations: CS, corticosteroids; DT, definitive therapy; HSCT, hematopoietic stem cell transplantation; IBD, inflammatory bowel disease; IL, interleukin; IMs, inflammatory manifestations; ND, nondeterminable; Pt, patient.
Clinical features of patients. (A-F) Inflammatory skin manifestations of patients. (A-B) Inflammatory skin rash in patient 5 at the onset of systemic inflammatory symptoms at 4 weeks of life (pre-HSCT). (C) Cellulitis-like lesions of the third finger in patient 8 (pre–gene therapy [GT]). (D) Vasculitic lesions of the trunk and the extremities in patient 1 at 2.5 months of age (pre-HSCT). (E) Pyoderma gangrenosum in patient 4 (pre-HSCT). (F) Vasculitis-like lesions of the foot in patient 2 (post-HSCT; see also panel G). (G) Post-HSCT donor chimerism and time course of inflammatory manifestations in patient 2.
Clinical features of patients. (A-F) Inflammatory skin manifestations of patients. (A-B) Inflammatory skin rash in patient 5 at the onset of systemic inflammatory symptoms at 4 weeks of life (pre-HSCT). (C) Cellulitis-like lesions of the third finger in patient 8 (pre–gene therapy [GT]). (D) Vasculitic lesions of the trunk and the extremities in patient 1 at 2.5 months of age (pre-HSCT). (E) Pyoderma gangrenosum in patient 4 (pre-HSCT). (F) Vasculitis-like lesions of the foot in patient 2 (post-HSCT; see also panel G). (G) Post-HSCT donor chimerism and time course of inflammatory manifestations in patient 2.
All patients received treatment with systemic corticosteroids, with only partial or no response in most of them. Anti–IL-1 agents (anakinra or canakinumab) led to good or complete responses in all patients. In most patients, the treatment effect was rapid (days); this was particularly evident in the 3 patients presenting with severe systemic inflammatory syndrome in the first months of life, in whom prompt and complete resolution of IMs, fever, and systemic symptoms was observed immediately after the commencement of anakinra. Good responses were observed also in the other patients, leading to the resolution of IMs often refractory to several other therapies (eg, pyoderma gangrenosum in patients 4 and 9). Therapy was also associated with normalization or near normalization of inflammatory markers (supplemental Table 2). In all patients, anti–IL-1 agents were used in addition to corticosteroids, allowing their tapering; in 5 patients, corticosteroids could eventually be discontinued, and IMs were controlled by anti–IL-1 agents alone. Notably, in most patients, treatment with anti–IL-1 agents had to be administered continuously and could not be withheld because symptoms tended to relapse (most evident in patient 5, in whom symptoms recurred within hours of a delayed dose of anakinra). Therapy was well tolerated in all patients, even for long periods (up to 8 years in 1 patient), allowing successful bridging to definitive treatment. Most commonly reported adverse effects included injection site lipohypertrophy and injection anxiety with anakinra, which improved after switching to canakinumab. Two patients developed hypereosinophilia: in 1 patient (patient 6), this was considered to be related to immune reconstitution after gene therapy (GT) and resolved spontaneously; in the second patient (patient 8), anakinra was briefly stopped as a precaution, and hypereosinophilia was successfully treated with low-dose corticosteroids; anakinra was then resumed, in association with low-dose corticosteroids, and continued until GT. Colchicine was used in 2 patients: in 1 patient, it led to near-complete resolution of cutaneous vasculitis symptoms for >1 year, yet it eventually lost its efficacy; in the second patient, it was added to long-standing anakinra, so it was difficult to determine its contribution; nevertheless, colchicine was stopped after 5 years because it was believed to be not helping.
All patients underwent definitive treatment for WAS, either by GT (4 patients) or HSCT (5 patients; details in Table 2 and supplemental Table 1). Treatment with anti–IL-1 agents was discontinued before HSCT/GT in all patients, except in 2 patients (patients 6 and 9), in whom anakinra was discontinued only after GT. Of 5 patients who underwent HSCT, 4 developed low (0%-30%) myeloid donor chimerism, with relatively preserved lymphoid chimerism (80%-95%). These patients experienced post-HSCT IMs, either for the first time (patients 2 and 3) or as a recurrence of pre-HSCT symptoms (patients 1 and 5). In 3 patients (patients 1, 2, and 5; Figure 1G), loss of myeloid chimerism was temporally associated with the occurrence of IMs, whereas in patient 3, post-HSCT IMs occurred in the setting of long-standing low myeloid chimerism. Two patients (patients 1 and 2) underwent a second HSCT, resulting in complete donor chimerism and resolution of IMs, whereas in the other 2 patients (patients 3 and 5), therapy with anakinra was continued; both patients were eventually switched to canakinumab because of frequent injection intolerance. Patients who underwent GT maintained a stable myeloid correction, and no patient experienced a recurrence of IMs.
Details of definitive therapy for Wiskott-Aldrich syndrome in patients
| Patient no. . | DT . | Declining donor chimerism after HSCT (% donor) . | IMs occurrence after DT? (% donor myeloid cells at IMs onset) . | 2nd HSCT . | Current status and FU duration after last DT . |
|---|---|---|---|---|---|
| 1 | HSCT (twice) | Yes, after 1st HSCT (CD3, 85%; CD14, 1%) | Yes, recurrence (CD14, 1%) | Yes | CR after 2nd HSCT, full donor chimerism (3 y FU) |
| 2 | HSCT (twice) | Yes, after 1st HSCT (CD3, 90%; CD15, 0%) | Yes, 1st appearance (CD15, 0%) | Yes | CR after 2nd HSCT, full donor chimerism (4 y FU) |
| 3 | HSCT | Yes, after HSCT (CD3, 80%; CD14, 30%) | Yes, 1st appearance (CD14, 30%) | No | IMs well-controlled on canakinumab |
| 4 | HSCT | No | No | No | CR after HSCT, full donor chimerism (3 y FU) |
| 5 | HSCT | Yes, after HSCT (CD3, 93%; CD15, 15%) | Yes, recurrence (CD15, 40%) | No | IMs well-controlled on canakinumab |
| 6 | GT | NA | No | No | CR (5 y FU) |
| 7 | GT | NA | No | No | CR (4.4 y FU) |
| 8 | GT | NA | No | No | CR (4.1 y FU) |
| 9 | GT | NA | No | No | CR (8 y FU) |
| Patient no. . | DT . | Declining donor chimerism after HSCT (% donor) . | IMs occurrence after DT? (% donor myeloid cells at IMs onset) . | 2nd HSCT . | Current status and FU duration after last DT . |
|---|---|---|---|---|---|
| 1 | HSCT (twice) | Yes, after 1st HSCT (CD3, 85%; CD14, 1%) | Yes, recurrence (CD14, 1%) | Yes | CR after 2nd HSCT, full donor chimerism (3 y FU) |
| 2 | HSCT (twice) | Yes, after 1st HSCT (CD3, 90%; CD15, 0%) | Yes, 1st appearance (CD15, 0%) | Yes | CR after 2nd HSCT, full donor chimerism (4 y FU) |
| 3 | HSCT | Yes, after HSCT (CD3, 80%; CD14, 30%) | Yes, 1st appearance (CD14, 30%) | No | IMs well-controlled on canakinumab |
| 4 | HSCT | No | No | No | CR after HSCT, full donor chimerism (3 y FU) |
| 5 | HSCT | Yes, after HSCT (CD3, 93%; CD15, 15%) | Yes, recurrence (CD15, 40%) | No | IMs well-controlled on canakinumab |
| 6 | GT | NA | No | No | CR (5 y FU) |
| 7 | GT | NA | No | No | CR (4.4 y FU) |
| 8 | GT | NA | No | No | CR (4.1 y FU) |
| 9 | GT | NA | No | No | CR (8 y FU) |
Abbreviations: CR, complete remission; FU, follow-up; GT, gene therapy; NA, not applicable.
The present clinical experience shows that several inflammatory manifestations of WAS are dependent on IL-1 and respond to its pharmacologic blockade, thus suggesting an autoinflammatory contribution to their pathogenesis. Although loss of tolerance because of abnormal T- and B-cell function has been clearly demonstrated in WAS,3,6,14-16 our experience underlines the role of inflammatory mechanisms in this condition. WAS protein (WASP) has a major role in leukocyte chemotaxis/activation,17,18 autophagy, and inflammasome activity.9 Furthermore, mutations in several WASP-interacting, cytoskeleton-related, proteins are associated with autoinflammatory disorders that have been included in a new group of immune defects with autoinflammation called “immune actinopathies” (eg, PSTPIP1, CDC42).19,20 Notably, similarly to other monogenic autoinflammatory conditions, some of our patients presented with an early-onset, sepsis-like, severe systemic inflammatory syndrome, whereas in others, IMs appeared at an older age and had a more chronic course. In all cases, IMs responded well to anti–IL-1 agents, which often could not be discontinued because of the reappearance of symptoms. Incomplete response to corticosteroids, especially if compared with direct IL-1 blockade, is consistent with what is observed in other monogenic autoinflammatory diseases. IL-1 exerts positive feedback on its production; thus, its direct blockade can be more effective than reduced transcription by corticosteroids. Furthermore, corticosteroids also suppress IL-1 receptor antagonist synthesis, resulting in reduced overall inhibitory efficacy.21 Colchicine, a drug that reduces IL-1b release through inhibition of caspase-1 and of NLR family, pyrin domain-containing 3 (NLRP3) inflammasome oligomerization, was also used in 2 patients, with mixed results; however, our data are too limited to draw conclusions on its efficacy.
The fact that several patients experienced IMs with declining myeloid (but not lymphoid) chimerism after HSCT suggests a central role for myeloid cells in autoinflammation in WAS. WASP-deficient myeloid cells exhibit impaired polarization, migration, and phagocytosis, but also increased activation of the NLRP3 inflammasome and IL-1b release following chemical or bacterial stimulation.9,22 Furthermore, WASP-deficient neutrophils are prone to spontaneous neutrophil extracellular trap release.23 The role of preserved lymphoid chimerism in this setting is uncertain; however, the fact that IMs occurred both before and after HSCT in patients with loss of myeloid chimerism suggests a more prominent role of defective myeloid cells. No patient experienced a relapse of IMs after GT, possibly because of more stable myeloid correction (supplemental Figure 1).
Therapy with anti–IL-1 agents was well tolerated in all patients, without severe adverse effects. This is consistent with their overall record of safety from other conditions. Anti–IL-1 therapies do not have direct organ toxicities, and they are not directly immunosuppressive, nor increase the risk of opportunistic infection; however, they may be associated with a slight increase of severe infections, possibly because of a blunting effect on infection signs and symptoms, leading to delayed diagnoses.24,25 On the other hand, treatment with anti–IL-1 therapies allowed corticosteroid sparing in all patients, therefore reducing their immunosuppressive effect. Overall, we do not suggest adjunctive anti-infectious therapies in patients treated with anti–IL-1 agents, yet the possibility of a more blunted clinical response to infection should be considered.
This study has several limitations, including its retrospective nature; therefore, a reporting bias cannot be excluded. We did not study molecular markers that could help identifying patients most likely to benefit from anti–IL-1 agents; therefore, at present, in consideration of its general safety and short half-life, a therapeutic trial of anakinra could represent the most effective strategy in patients presenting with refractory IMs. Overall, our experience encourages further studies on the use of anti–IL-1 agents as first-line treatment for inflammatory manifestation in patients with WAS, possibly in lieu of other immunosuppressive drugs often associated with a less favorable safety profile.
Acknowledgments
This work was supported by the Italian Ministero della Salute (Rome, Italy) in collaboration with the Institute for Maternal and Child Health IRCCS Burlo Garofolo (Trieste, Italy) with grant RC 30/22; and with grant Ricerca Finalizzata GR-2016-02365089 “Role of follicular helper T cells in the humoral immune alterations in patients with mutations in the Wiskott Aldrich syndrome protein before and after hematopoietic stem cell transplantation”; and by the European Union/Next Generation EU Fund “Implementation of an Italian Network for advanced diagnosis and targeted treatment of inborn errors of immunity” (PNRR MR1-2022-12376594) and “Immunogenicity and long-term memory maintenance of SARS-CoV2 mRNA vaccination in patients affected by inborn errors of immunity” (PNRR M4C2-2022-ZCLC3X).
Authorship
Contribution: S.N. designed the study, cared for patients, collected and analyzed data, and wrote the first draft of the manuscript; M.P.C., E.R., F.F., A.J.T., and A.A. designed the study, cared for patients, and critically reviewed the manuscript; and C.B., S.C., K.-N.C., M.F., S. Giardino, S. Ghosh, P.P.L., P.T.L., R.M., V.S., A. Tessitore, A. Tommasini, E.V., T.C.V., S.V., A.J.W., M.R., and M.H.A. cared for patients, collected data, and critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Samuele Naviglio, Institute for Maternal and Child Health IRCCS “Burlo Garofolo,” Trieste, Italy; email: samuele.naviglio@burlo.trieste.it.
References
Author notes
S.N., M.P.C., and E.R. contributed equally to this study.
For original data, please contact Samuele Naviglio (see “Correspondence”).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Clinical features of patients. (A-F) Inflammatory skin manifestations of patients. (A-B) Inflammatory skin rash in patient 5 at the onset of systemic inflammatory symptoms at 4 weeks of life (pre-HSCT). (C) Cellulitis-like lesions of the third finger in patient 8 (pre–gene therapy [GT]). (D) Vasculitic lesions of the trunk and the extremities in patient 1 at 2.5 months of age (pre-HSCT). (E) Pyoderma gangrenosum in patient 4 (pre-HSCT). (F) Vasculitis-like lesions of the foot in patient 2 (post-HSCT; see also panel G). (G) Post-HSCT donor chimerism and time course of inflammatory manifestations in patient 2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/144/16/10.1182_blood.2024024524/3/m_blood_bld-2024-024524-gr1.jpeg?Expires=1769082115&Signature=p3ZOQqD6bkcgMXHyiUZ~NHjQGoUf95u9ZIdRLAdeJi2dT6XF2OhX-xIs7TSEtAMj4XFdYVbIZ4ZcQBXsIGwYCXqyZNtIil~JIAvIkS01EdRT-9yhwuE7~8nE09zJXehbqc89gecxGGJ8waYBGkf5jTNeJhSjMsgx5uWdaP0sJAOGl2Y-1J50xhoILdqUF~PnlJiIpGcv709Pjj2AFLlFjimLj1FWRlP5dlCt72zrBgr7k3c30QI6B7SYB00I2yNazCQgFDzpZRpzMx1H2z7D5MgxYTnJbd~KHbNltyUpW4q8Dg2XwyGTSUs7plPAYyyU4lcYo9N-lr5jpDcMAxSo~w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal