Key Points
JAK2-mutant clonal hematopoiesis is associated with a strong risk of VTE.
JAK2-mutant CHIP confers a greater risk of VTE than heterozygous thrombophilia but is present at a lower frequency in the general population.
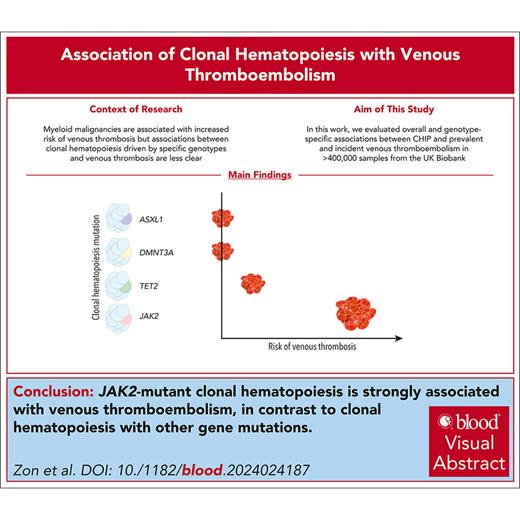
Visual Abstract
Venous thromboembolism (VTE) is common among older individuals, but provoking factors are not identified in many cases. Patients with myeloid malignancies, especially myeloproliferative neoplasms (MPNs), are at increased risk for venous thrombosis. Clonal hematopoiesis of indeterminate potential (CHIP), a precursor state to myeloid malignancies, is common among older individuals and may similarly predispose to venous thrombosis. We evaluated overall and genotype-specific associations between CHIP and prevalent and incident VTE in >400 000 samples from the UK Biobank. CHIP was modestly associated with incident VTE with a hazard ratio (HR) of 1.17 (95% confidence interval [CI], 1.09-1.3; P = .002) but was not significantly associated with prevalent VTE with an odds ratio (OR) of 1.02 (95% CI, 0.81-1.23; P = .81). TET2-mutant CHIP was associated with incident VTE with a HR of 1.33 (95% CI, 1.05-1.69; P = .02). JAK2 mutations were highly associated with both prevalent and incident VTE risk, with an OR of 6.58 (95% CI, 2.65-16.29; P = 4.7 × 10–5) and a HR of 4.2 (95% CI, 2.18-8.08; P = 1.7 × 10–5), respectively, consistent with the thrombophilia associated with JAK2-mutant MPN. The association between JAK2-mutant CHIP and VTE remained significant after excluding potential undiagnosed MPN based on laboratory parameters. JAK2-mutant CHIP was more strongly associated with VTE but was less common than heterozygous factor V Leiden and heterozygous prothrombin gene mutation. These results indicate that most individuals with CHIP do not have an altered risk of thrombosis, but individuals with JAK2-mutant CHIP have a significantly elevated risk of VTE.
Introduction
Venous thromboembolism (VTE) affects nearly 10 million people worldwide every year.1 Risk factors for VTE include recent major surgery, malignancy, and inherited thrombophilia. However, 25% to 50% of venous thromboembolic events do not have a clear, identifiable cause.2 Myeloid malignancies increase the risk of venous thrombosis, especially among those with myeloproliferative neoplasms (MPNs).3,4 Studies have shown a 10-fold increased risk of MPN-associated thrombotic events compared with healthy individuals.5-7
Clonal hematopoiesis of indeterminate potential (CHIP) is a premalignant state characterized by myeloid malignancy driver mutations with a variant allele fraction ≥0.02 in individuals without blood count abnormalities.8,9 CHIP increases in prevalence with age, occurring in ∼10% of individuals aged >70 years.10,11 CHIP is associated with an increased risk of hematologic malignancies and has also been associated with nonhematologic pathologies. These conditions, many of which are etiologically linked to inflammation, include cardiovascular disease,12,13 stroke,14 kidney disease,15,16 rheumatologic conditions,17 and liver disease.18
Studies evaluating the relationship between CHIP and VTE have arrived at conflicting results. Retrospective studies have found prevalent CHIP in 19% of patients with unprovoked pulmonary embolism,19 37.8% and 46% in patients with splanchnic vein thrombosis,20,21 and 3.7% in patients with unprovoked proximal VTE.22 In a larger cohort study, in which 45% of individuals had schizophrenia, incident venous thrombosis rates in patients with CHIP were 5%, compared with 2.1% in patients without CHIP.23 Examination of genotype-specific associations between CHIP and VTE requires substantially larger cohorts. We, therefore, examined CHIP and VTE in the UK Biobank, a study with >400 000 individuals linked to exome sequencing of peripheral blood DNA and detailed clinical phenotypes.
Methods
UK Biobank and analyses
A total of 502 490 individuals from the UK Biobank were evaluated for inclusion in this study. Data were obtained for these participants through UK Biobank (application number 50834). Individuals with whole-exome sequencing data and nonmissing data across all covariates were included. Individuals were excluded if genomic analyses did not pass quality control, individuals were outliers for heterozygosity or missing rate, the individual had a prevalent diagnosis of a hematologic malignancy (list of diagnosis codes as previously described24) before DNA sampling or within 6 months after enrollment in the study, or consent was withdrawn.
VTE events were identified based on International Classification of Diseases (ICD10) codes (supplemental Table 1, available on the Blood website). Prevalent and incident VTE cases were ascertained with respect to the time of DNA sampling. Prevalent VTE was defined as having a VTE at or before the time of DNA sampling. An incident VTE was defined as an event that happened after DNA sampling. Median follow-up time for incident VTE was 11.8 years. Associations of CHIP and VTE were assessed using logistic regression for prevalent analyses and Cox proportional hazards for incident analyses. For the incident analysis, we excluded those with prevalent VTE at the time of DNA sampling. The association analyses were adjusted for age at the time of DNA sampling, age2, sex, European ancestry, first 5 genetic principal components, smoking status (ever vs never), and body mass index as covariates. Analyses were conducted in R Studio.
CHIP detection
CHIP mutations were identified as described previously24 using whole-exome sequencing data from blood DNA from UK Biobank participants. Somatic variants in genes associated with clonal hematopoiesis and/or myeloid malignancies were detected using Mutect2.10,25-27 The list of genes in which pathogenic mutations were identified for CHIP calling, and gene-level sequencing coverage statistics for this cohort are described elsewhere.28 The variant allele fraction had to be at least 0.02 to be considered a CHIP mutation. A minimum total read depth of 20 and a minimum read depth of 5 supporting the alternative allele were required. To minimize likelihood of detecting germ line variants and artifacts, the Genome Aggregation Database was used as a germ line reference, and a panel-of-normal derived from the youngest participants in the UK Biobank cohort was used.
Definition of potential undiagnosed cases of MPN
With the inclusion of a JAK2 mutation, laboratory findings suggestive of MPN were defined as polycythemia vera with hemoglobin >16.5 g/dL for males or hemoglobin >16 g/dL for females or essential thrombocytosis with platelets >450 × 109/L based on the International Consensus Classification.29JAK2 mutations in this analysis were all V617F.
Association of VTE with heterozygous inherited thrombophilias
Association of VTE with factor V Leiden (heterozygous) and prothrombin gene mutation (heterozygous) was analyzed in the UK Biobank based on single nucleotide polymorphism genotypes for rs6025 and rs1799963, respectively. The risk associated with heterozygous factor V Leiden or prothrombin gene mutation was compared with that of individuals who were homozygous wild type (individuals with homozygous factor V Leiden mutation or homozygous prothrombin mutation were not included in the analysis given their low frequency). Association was analyzed in a logistic regression model with VTE as the binary outcome variable and single nucleotide polymorphism genotype as the predictor variable with covariates of age, age2, sex, body mass index, European ancestry, smoking status (ever vs never), and the first 5 genetic principal components as covariates.
Results
Analysis of CHIP with incident VTE
Given the established association between myeloid neoplasms and VTE, we sought to determine whether a similar relationship existed between CHIP and VTE. Of 502 490 individuals in the UK Biobank, 425 399 individuals had high-quality exome sequencing, did not have prevalent hematologic malignancies by ICD10 codes, and had documented covariates. Among these individuals, the mean age of the participants was 56.6 years, 54% were female, 84% were of European ancestry, and 60% had ever smoked.
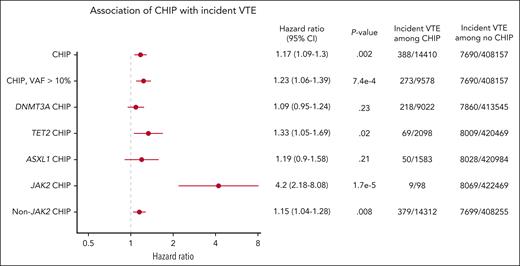
CHIP was modestly associated with incident VTE with a hazard ratio (HR) of 1.17 (95% confidence interval [CI], 1.09-1.3; P = .002), and CHIP with variant allele fraction (VAF) >10% had a HR of 1.23 (95% CI, 1.06-1.39; P = 7.4 × 10–4; Figure 1). We evaluated the 3 most commonly mutated genes in CHIP and found that TET2-mutant CHIP was modestly associated with incident VTE, with a HR of 1.33 (95% CI, 1.05-1.69; P = .02), but DNMT3A-mutant and ASXL1-mutant CHIP were not significantly associated with VTE.
Incidence of VTE in individuals with and without CHIP analyzed using Cox proportional hazards, corrected for age, sex, European ancestry, genetic principal components, ever smoked status, and body mass index.
Incidence of VTE in individuals with and without CHIP analyzed using Cox proportional hazards, corrected for age, sex, European ancestry, genetic principal components, ever smoked status, and body mass index.
Because JAK2-mutant MPNs are a potent risk factor for VTE, we hypothesized that JAK2-mutant CHIP may be particularly associated with an increased risk of VTE. Indeed, we found that JAK2-mutant CHIP was also strongly associated with incident VTE with a HR of 4.2 (95% CI, 2.18-8.08; P = 1.7 × 10–5). Among 98 individuals with JAK2-mutant CHIP, 9 had a documented incident VTE during a median of 6.98 years of follow-up (Figure 1). Given the strong association between JAK2-mutant CHIP and VTE and the fact that patients with JAK2-mutant MPNs have been found to have venous thrombotic events at unusual sites, such as splanchnic vein thromboses,30,31 we evaluated the types of venous thrombotic events seen in our cohort. In the 9 individuals with JAK2-mutant and incident VTE, 4 had deep vein thrombosis, 4 had pulmonary embolism, and 1 had portal vein thrombosis. In these 9 individuals, 6 developed an MPN after DNA sampling and before or at the time of incident VTE.
JAK2-mutant CHIP is strongly associated with prevalent VTE
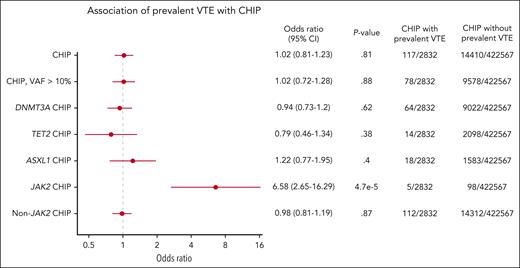
Because CHIP may exist for many years, we analyzed the frequency of CHIP in individuals with and without prevalent VTE (defined as a VTE event at or before DNA sampling). We again found that JAK2-mutant CHIP was strongly associated with prevalent VTE, with an odds ratio (OR) of 6.58 (95% CI, 2.65-16.29; P = 4.7 × 10–5). Among 2832 individuals with a prevalent VTE, 5 individuals had JAK2 mutations. Of these 5 individuals, 2 had deep vein thrombosis, 1 had pulmonary embolism, 1 had portal vein thrombosis, and 1 had Budd Chiari based on ICD10 codes. In contrast, there was no association found between prevalent VTE and CHIP overall (OR, 1.02; 95% CI, 0.81-1.23; P = .81) or CHIP overall with VAF >10% (OR, 1.02; 95% CI, 0.72-1.28; P = .88; Figure 2).
Prevalence of CHIP in individuals with and without VTE analyzed using logistic regression, corrected for age, sex, European ancestry, genetic principal components, ever smoked status, and body mass index.
Prevalence of CHIP in individuals with and without VTE analyzed using logistic regression, corrected for age, sex, European ancestry, genetic principal components, ever smoked status, and body mass index.
JAK2-mutant CHIP is strongly associated with VTE after excluding potential undiagnosed MPNs
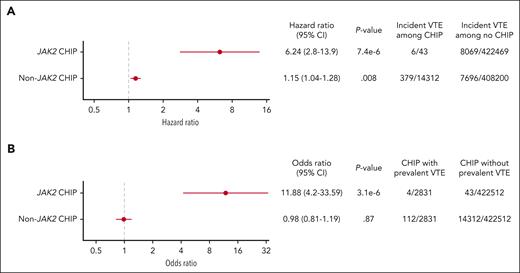
We next considered the possibility that the VTE association with JAK2-mutant CHIP could be driven by undiagnosed cases of MPNs that are not diagnosed or captured by ICD10 codes. To identify potential JAK2-mutated MPNs in the UK Biobank that were not identified based on ICD10 codes, we analyzed the elevations in laboratory values of hemoglobin and platelet counts using criteria established by the International Consensus Classification (supplemental Table 2).9 With MPN diagnoses excluded by both ICD10 codes and in those with cytoses, JAK2-mutant CHIP remained significantly associated with incident VTE with a HR of 6.24 and prevalent VTE with an OR of 11.88 (Figure 3A-B, respectively). Although those with cytopenias may represent advanced disease and would therefore be more likely to have a formal diagnosis, we further analyzed the association of JAK2-mutant CHIP with both incident and prevalent VTE when MPN diagnoses were excluded based on ICD10 codes, cytoses, and cytopenias; the HR for incident VTE with JAK2-mutant CHIP was 7.49, and the prevalent odds for VTE ratio was 14.59 (supplemental Figure 1).
Association between (A) JAK2-mutant CHIP and incident VTE and (B) JAK2-mutant CHIP and prevalent VTE with myeloid neoplasms excluded by ICD10 codes and potential cases of undiagnosed MPN excluded based on cytoses.
Association between (A) JAK2-mutant CHIP and incident VTE and (B) JAK2-mutant CHIP and prevalent VTE with myeloid neoplasms excluded by ICD10 codes and potential cases of undiagnosed MPN excluded based on cytoses.
Risk of VTE with JAK2-mutant CHIP relative to inherited heterozygous thrombophilias
Although JAK2-mutant CHIP was associated with a significantly increased risk of incident VTE (HR, 6.24), it accounted for only a small fraction (5/2832) of individuals with prevalent VTE (Table 1). The median follow-up time to VTE for JAK2-mutant CHIP was 6.98 years and for non-JAK2-mutant CHIP was 7.23 years. To contextualize these findings with well-established risk factors for VTE in the same cohort, we examined the risk of inherited thrombophilias. Using germ line genetic data in the UK Biobank, we identified individuals with factor V Leiden and prothrombin gene mutation. Heterozygous factor V Leiden and heterozygous prothrombin gene mutation G20210A had HRs of 2.36 and 1.91, respectively. The median age of VTE diagnosis for both heterozygous thrombophilias was 64.88 years. These studies demonstrate that the impact of JAK2-mutant CHIP on the risk of VTE may be greater than common heterozygous inherited thrombophilias, although the prevalence of JAK2-mutant CHIP is lower than these germ line risk alleles.
JAK2-mutant CHIP relative incident risk of VTE and prevalence in the general population compared with heterozygous factor V Leiden and heterozygous prothrombin gene mutation G20210A in the UK Biobank
| Thrombophilia . | HR of first episode of VTE compared with controls . | Prevalence in the population . |
|---|---|---|
| Heterozygous factor V Leiden | 2.36 (95% CI, 2.21-2.52) | 4.4% |
| Heterozygous prothrombin G20210A | 1.91 (95% CI, 1.74-2.10) | 2.3% |
| JAK2 CHIP | 6.24 (95% CI, 2.8-13.9) | 98/430 000 (0.02%) 0.14%-3% in other studies11,32-34 |
| Non-JAK2 CHIP | 1.15 (95% CI, 1.04-1.28) | 14 417/430 000 (3.4%) |
| Thrombophilia . | HR of first episode of VTE compared with controls . | Prevalence in the population . |
|---|---|---|
| Heterozygous factor V Leiden | 2.36 (95% CI, 2.21-2.52) | 4.4% |
| Heterozygous prothrombin G20210A | 1.91 (95% CI, 1.74-2.10) | 2.3% |
| JAK2 CHIP | 6.24 (95% CI, 2.8-13.9) | 98/430 000 (0.02%) 0.14%-3% in other studies11,32-34 |
| Non-JAK2 CHIP | 1.15 (95% CI, 1.04-1.28) | 14 417/430 000 (3.4%) |
Discussion
Our study demonstrates definitively that, in a large population-based cohort with whole-exome sequencing, JAK2-mutant CHIP is most strongly associated with both incident and prevalent VTE compared with other CHIP genotypes. Although other CHIP genotypes have been associated with inflammatory disorders, VTE is selectively associated with JAK2 mutations.10,12,35,36 Our findings validate reports from smaller cohorts, nonpopulation-based cohorts, or cohorts that had more limited genotyping of somatic mutations.23,37-39 The relationship between JAK2-mutant CHIP and VTE likely involves multiple mechanisms including increased release of neutrophil extracellular traps and impacts on platelets and red blood cells.23,32JAK2-mutant CHIP was also recently found to increase the risk of arterial thrombosis due to younger and more activated platelets.33
The UK Biobank cohort enabled us to examine the strength of association between JAK2-mutant CHIP and VTE, as well as the impact of germ line predisposition to VTE in the same population. Similarly, the relative prevalence of these germ line and somatic risk factors was assessed in the same cohort. Somatic JAK2 mutations carry a higher risk of thrombosis in the UK Biobank than inherited mutations in the factor V or prothrombin genes. On the contrary, somatic JAK2 mutations were much rarer than the major inherited thrombophilias. The prevalence of JAK2-mutant CHIP in our study was 0.02%, which is lower than prior documented rates ranging from 0.14% to 3.1% depending on the depth of sequencing coverage.11,37-39 The exome sequencing data in the UK Biobank had lower coverage of the JAK2 locus than other CHIP genes, thereby decreasing the sensitivity of detection for smaller JAK2-mutant clones. It is likely that the VTE risk from smaller JAK2 clones is lower than the risk associated with larger clones, as has been demonstrated for other CHIP-associated phenotypes.10,12,34 A Danish population study of ∼20 000 individuals showed an OR for prevalent VTE of 2.8 in individuals with JAK2 VAF ≥0.01 vs an OR of 0.71 with JAK2 VAF <0.01, further supporting the increased risk of VTE with larger JAK2 clone sizes as found in our cohort.38 Using an estimated prevalence of JAK2 V617F of 2 of 1000 in the general population based on the middle of the range from our study and others11,37-39 and ∼77 million individuals aged >60 years in the United States,40 154 000 individuals in the United States would be estimated to have JAK2-mutant CHIP.34
Our findings demonstrate that, among individuals with CHIP, those with JAK2-mutant CHIP have a striking risk of developing a VTE. Although we do not recommend screening for JAK2 mutations, particularly due to the low prevalence of JAK2-mutant CHIP, the index of suspicion may be increased by blood count parameters or VTE location. In the UK Biobank, individuals with CHIP who developed a VTE event tended to have higher hemoglobin and/or platelet counts, even though values were within the normal range and would therefore not be consistent with a diagnosis of MPN based on these parameters. In addition, individuals with JAK2-mutant CHIP may have VTE events in unusual locations, such as splanchnic vein thrombosis, based on data from the UK Biobank and prior publications.41,42 Even without screening, widespread use of next-generation sequencing panels has resulted in an increased number of individuals diagnosed with JAK2-mutant CHIP. Future studies will aid in determining approaches to VTE risk reduction for individuals with JAK2-mutant CHIP.
Acknowledgments
R.L.Z. is supported by the American Society of Hematology Research Training Award for Fellows grant and the Mark Foundation for Cancer Research Physician-Scientist of the Damon Runyon Cancer Research Foundation (PST-44-24). B.L.E. is supported by the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (grant R01HL082945; NIH, National Cancer Institute (grants P01CA108631, P50CA206963, and R35CA253125), the Howard Hughes Medical Institute, and the Fondation Leducq. A.G.B. is supported by the NIH Office of the Director (grant DP5 OD029586), a Burroughs Wellcome Fund Career Award for Medical Scientists, the E.P. Evans Foundation, and a Pew-Stewart Scholar for Cancer Research award. A.N. was supported by funds from Knut and Alice Wallenberg Foundation (KAW2017.0436) and the SciLifeLab & Wallenberg Data Driven Life Science Program (KAW 2020.0239).
Authorship
Contribution: R.L.Z., A.S., and B.L.E. designed and performed the research, analyzed the data, and wrote the manuscript; K.C. performed the research, contributed data for the factor V Leiden and prothrombin gene mutation analyses, and contributed analytical tools; K.C., O.O., A.N., A.B., C.J.G., G.G., M.M.U., and P.N. contributed analytical tools; and D.N. performed the research and analyzed the data.
Conflict-of-interest disclosure: B.L.E. has received research funding from Celgene, Deerfield, Novartis, and Calico; has received consulting fees from AbbVie and GRAIL; and is a member of the scientific advisory board and shareholder for Neomorph Inc, TenSixteen Bio, Skyhawk Therapeutics, and Exo Therapeutics. P.N. reports research grants from Allelica, Apple, Amgen, Boston Scientific, Genentech/Roche, and Novartis; personal fees from Allelica, Apple, AstraZeneca, Blackstone Life Sciences, Creative Education Concepts, CRISPR Therapeutics, Eli Lilly & Co, Esperion Therapeutics, Foresite Labs, Genentech/Roche, GV, HeartFlow, Magnet Biomedicine, Merck, Novartis, TenSixteen Bio, and Tourmaline Bio; equity in Bolt, Candela, Mercury, MyOme, Parameter Health, Preciseli, and TenSixteen Bio; and spousal employment at Vertex Pharmaceuticals, all unrelated to the present work. A.B. is on the scientific advisory board membership at TenSixteen Bio. K.C. is on the advisory board for United Therapeutics and receives consulting fees from Amgen, Tectonic Therapeutic, and United Therapeutics. A.S. reports stock in Vertex. R.L.Z. is a stockholder in and receives consultancy fees from Triveni Bio. The remaining authors declare no competing financial interests.
Correspondence: Benjamin L. Ebert, Dana-Farber Cancer Institute, 450 Brookline Ave, D1610A, Boston, MA 02215; email: benjamin_ebert@dfci.harvard.edu.
References
Author notes
R.L.Z. and A.S. contributed equally to this study.
The patient cohort used for this study is the UK Biobank. The source data are available to the approved researchers through the UK Biobank. Individual-level UK Biobank data are available for approved researchers at https://www.ukbiobank.ac.uk. This cohort has been used in multiple other studies, available at https://doi.org/10.1038/s41591-021-01521-4 and https://doi.org/10.1038/s41408-023-00974-9.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal