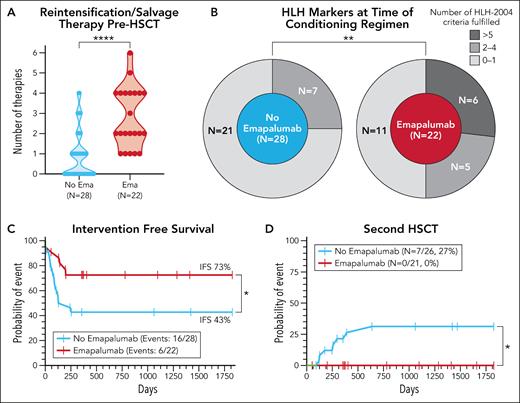
In this issue of Blood, Verkamp and colleagues1 present a retrospective analysis of the emapalumab (anti-interferon gamma [IFN-γ] monoclonal antibody) use before reduced-intensity conditioning (RIC) allogeneic hematopoietic stem cell transplantation (HSCT) in children with HLH (hemophagocytic lymphohistiocytosis). Although a survival benefit was not demonstrated, a lower incidence of mixed chimerism (48% vs 77%, P = .03) and a higher intervention-free survival (IVS) (73% vs 43%, P = .03) occurred after using emapalumab. These findings are strengthened by the observation that children in the emapalumab group had higher HLH activity as shown by HLH markers before the conditioning and more often presented with a refractory disease (see figure panels A-C).
Selected patient characteristics and transplant outcomes. (A) Number of previous therapies. (B) Number of HLH-2004 criteria fulfilled at the time of conditioning. (C) IFS. (D) Probability of second HSCT. ∗∗∗∗P < .0001; ∗∗P < .01; ∗P < .05. Ema, emapalumab. See Figures 1, 3, and 4 in the article by Verkamp et al that begins on page 2625. Professional illustration by Patrick Lane, ScEYEnce Studios.
Selected patient characteristics and transplant outcomes. (A) Number of previous therapies. (B) Number of HLH-2004 criteria fulfilled at the time of conditioning. (C) IFS. (D) Probability of second HSCT. ∗∗∗∗P < .0001; ∗∗P < .01; ∗P < .05. Ema, emapalumab. See Figures 1, 3, and 4 in the article by Verkamp et al that begins on page 2625. Professional illustration by Patrick Lane, ScEYEnce Studios.
HLH is a state of intense, lethal inflammation where a cytokine storm is started by infection, malignancy, or autoimmune disease. In the presented analysis, patients developed HLH at an early age (median at transplantation was <18 months), because of a confirmed mutation in HLH-causing genes (primary HLH) in 86% of cases. The only cure for these patients with primary HLH is allogeneic HSCT.
Myeloablative conditioning (MAC) was used in the first allogeneic HSCT for HLH in 19842 and continued as the standard in HLH-94 and HLH-20043 protocols. Unfortunately, a full preparative regimen caused significant toxicity, which negatively impacted survival. Marsh and colleagues first reported the advantage of RIC in a direct comparison with MAC in the single-center pediatric HLH setting.4 It is a logical approach in a syndrome where there is no need to destroy malignant cells but to restore the function of the immune system. Unfortunately, the major drawback of RIC is mixed chimerism and graft rejection, requiring careful medical management including up to second transplantations. This problem was the focus of the current study. No second transplants were needed in the emapalumab group compared with 27% in the standard treatment group (see figure panel D). Emapalumab helps transplanted stem cells feel at home.
The authors conducted a retrospective study of pediatric patients with HLH receiving a first RIC HSCT at a single institution between 2014 and 2022 after treatment for HLH, with or without emapalumab. The end points examined included mixed chimerism (<95% donor chimerism), severe mixed chimerism (<25% donor) chimerism, and IFS (including donor lymphocyte infusion, infusion of donor CD34-selected cells, second HSCT, or death within 5 years post-HSCT). Of the 50 included patients, 22 received emapalumab within 21 days prior to the conditioning regimen and 28 did not. Use of emapalumab was associated with a markedly lower incidence of mixed chimerism (48% vs 77%, P = .03) and severe mixed chimerism (5% vs 38%, P < .01). IFS was similarly impacted and was significantly higher in patients receiving emapalumab (73% vs 43%, P = .03). This improvement was more striking in infants <12 months, a group at the highest risk for mixed chimerism (75% vs 20%, P < .01). Although overall survival was higher with emapalumab, this difference did not reach statistical significance (82% vs 71%, P = .39).
An alternative approach to enhancing HSCT results in pediatric HLH may still be using MAC, where event-free survival may reach 100%.2 HLH transplants in adults, although less frequent than in the pediatric setting, are in great need of advancements as the historical results are much worse than in children: 3- and 5-year survival of 44% (23% above 30 years of age), with no difference between RIC and MAC.5 Unfortunately, results in Verkamp and colleagues’ pediatric cohort also deteriorate with age and are worse for patients older than only 12 months, which mitigates the hope of seamless translation to adults.
Emapalumab was designed as the first drug intended for HLH treatment.6 The initial observation that IFN-γ plays a crucial role in the HLH cytokine storm was elegantly translated into a new treatment option. Here, we can see its properties, which could potentially be transferred to a transplant setting for other diseases. Future studies could also explore the use of emapalumab in other contexts. IFN-γ is an instigator of unwanted inflammatory reactions besides HLH. For example, would blockade of IFN-γ reduce incidence or severity of acute graft-versus-host disease by reducing the other cytokines produced as a reaction to the toxicity from the preparative regimen? Similarly, would it reduce toxicity from cytokine release syndrome from chimeric antigen receptor T cells? These states are also mediated by IFN-γ, which, binding with emapalumab, could have a positive effect.7,8
Given the limitations of this single-center, retrospective study, more analyses are needed in this setting. The lack of emapalumab approval in Europe (by the European Medicines Agency) limits the generalizability of presented findings. Although a statistically significant change in overall survival was not achieved in this small study, a lower need for interventions (including those as important as second transplants) has a clear positive impact. Patients can stay longer at home when we help transplanted stem cells feel at home.
Conflict-of-interest disclosure: R.M. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal