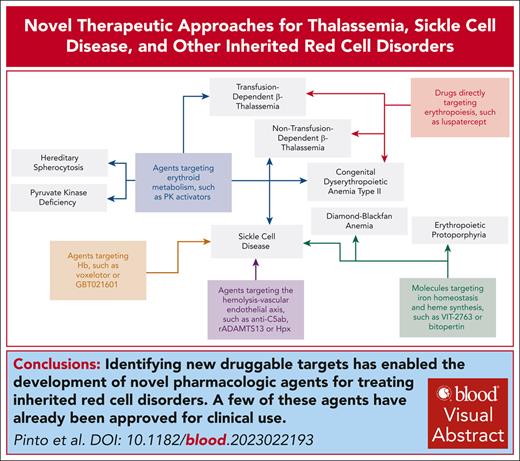
Visual Abstract
In this last decade, a deeper understanding of the pathophysiology of hereditary red cell disorders and the development of novel classes of pharmacologic agents have provided novel therapeutic approaches to thalassemias, sickle cell disease (SCD), and other red cell disorders. Here, we analyze and discuss the novel therapeutic options according to their targets, taking into consideration the complex process of erythroid differentiation, maturation, and survival of erythrocytes in the peripheral circulation. We focus on active clinical exploratory and confirmatory trials on thalassemias, SCD, and other red cell disorders. Beside β-thalassemia and SCD, we found that the development of new therapeutic strategies has allowed for the design of clinic studies for hereditary red cell disorders still lacking valuable therapeutic alternative such as α-thalassemias, congenital dyserythropoietic anemia, or Diamond-Blackfan anemia. In addition, reduction of heme synthesis, which can be achieved by the repurposed antipsychotic drug bitopertin, might affect not only hematological disorders but multiorgan diseases such as erythropoietic protoporphyria. Finally, our review highlights the current state of therapeutic scenarios, in which multiple indications targeting different red cell disorders are being considered for a single agent. This is a welcome change that will hopefully expand therapeutic option for patients affected by thalassemias, SCD, and other red cell disorders.
Introduction
The global burden of disease for thalassemias, sickle cell disease (SCD), and red cell disorders has emerged as a significant public health problem due to the negative impact on quality of life and survival.1-3 A better understanding of the pathophysiology of these disorders has allowed for the identification of new druggable targets.4-6 In this review, we analyzed active clinical exploratory and confirmatory trials on thalassemias, SCD, and other red cell disorders. We exclude (1) paroxysmal nocturnal hemoglobinuria and autoimmune hemolytic anemias7,8; (2) therapeutic strategies for ineffective erythropoiesis in low-grade myelodysplastic syndrome9; and (3) fetal hemoglobin (Hb) inducers because they were recently reviewed in SCD,10 and a new review on reactivation of fetal Hb synthesis will be part of this series of reviews. The novel therapeutic tools are discussed according to their targets and taking into consideration the complex process of erythroid differentiation, maturation, and survival of erythrocytes in the peripheral circulation.
Drug directly targeting erythropoiesis: luspatercept
Erythropoiesis is a dynamic and complex process going from committed erythroid progenitors throughout erythroblasts maturation to reticulocyte and mature red cells. After the approvals of multiple erythropoietic stimulating agents, luspatercept, a ligand trap with high affinity for activin B, is the newest addition targeting both normal and pathologic erythropoiesis. The serendipitous observation of increased Hb in healthy women treated with luspatercept for postmenopausal bone disease opened the way to its consideration for red cell disorders. Studies in mouse models for β-thalassemia have shown that luspatercept action goes beyond activin B, possibly sequestrating multiple activin-receptor ligands such as growth differentiation factor 11 or bone morphogenetic proteins.4,11-13 Thus, luspatercept has been proposed to indirectly affect (transforming growth factor β)–suppressor of mothers against decapentaplegic canonical pathways, possibly rebalancing the intracellular cell signaling and favoring erythroid maturation in late phase of erythropoiesis.4,14,15 Luspatercept was evaluated in transfusion-dependent (BELIEVE [NCT02604433]) and non–transfusion-dependent (BEYOND [NCT03342404]) patients with β-thalassemia. Luspatercept significantly improved transfusion burden and anemia in transfusion-dependent thalassemias (TDTs) and non–transfusion-dependent thalassemias (NTDTs), respectively, with good safety and tolerability profile (BELIEVE and BEYOND studies, respectively; supplemental Table 1, available on the Blood website).15-19 Although the BEYOND in NTDT study did not achieve the secondary end point of patient-reported outcomes, the improvement of symptomatic anemia and the lack of valuable therapeutic alternative supported the approval of luspatercept for the treatment of patients with NTDT by European Medicines Agency (EMA). This is different from the Food and Drug Administration (FDA) position, which highlights the absence of agreement on benefit and risk of treatment of this heterogeneous patient population.4,16
More recently, Wobus et al reported20 modulation of mesenchymal cell function, suggesting a possible action of luspatercept also in the early stage of erythropoiesis. These data have allowed for the design of clinical studies to evaluate the effect of luspatercept in the following patients populations: (1) pediatric β−thal/Hb E (NCT04143724); (2) α-thalassemia HbH (NTDT e TDT [NCT05664737]); and (3) congenital dyserythropoietic anemia type II (CDAII; EudraCT number 2020-005736-30) (Table 1; supplemental Table 2).
Ongoing clinical studies in thalassemias, SCD, and other red cell disorders
| Mechanism . | Agent . | Study title/NCT/populations . | Clinical phase . | Status . |
|---|---|---|---|---|
| Drug directly targeting erythropoiesis | Luspatercept | NCT04143724 Population: β-thal or HbE/β-thal Age: 6-17 y Enrollment (estimated) N = 54 pts | Phase 2 A phase 2a study of the safety and pharmacokinetics of luspatercept (ACE-536) in pediatric participants under regular transfusions due to (β)-thalassemia | Recruiting |
| NCT05664737 Population: α-thal HbH disease Age: ≥18 y Enrollment (estimated) N = 177 pts | Phase 2 A phase 2, double-blind, randomized, placebo-controlled, multicenter study to determine the efficacy and safety of luspatercept (BMS-986346/ACE-536) for the adults with alpha (α)-thalassemia | Recruiting | ||
| EudraCT number: 2020-005736-30 Population: CDAII (biallelic causative mutations in SEC23B gene alone or associated with other gene variants or monoallelic causative mutations in SEC23B gene in presence of hypoglycosylation of band 3 and/or associated with other gene variants) Age: ≥18 y Enrollment (estimated) N = 40 pts | Phase 2 A phase 2, multicenter, open-label study. Efficacy and safety of luspatercept (ACE-536) in adult patients with CDA II | Active, not recruiting | ||
| Targeting iron homeostasis or heme synthesis | FPN blocker | ViSionSerenity (NCT04817670) Population: HbSS or HbS/β0 thal Age:18-60 y Enrollment (estimated) N = 24 pts | Phase 2 Double-blind, randomized, placebo-controlled, efficacy, and safety study of multiple doses of VIT-2763 in subjects with SCD | Recruiting |
| Bitopertin | NCT05828108 Population: steroid-refractory DBA Age: 18-100 y Enrollment (estimated) N = 30 pts | Phase 1/2 Intrapatient dose-escalation study of the selective GlyT1 inhibitor bitopertin for steroid-refractory Diamond-Blackfan anemia | Recruiting | |
| BEACON (ACTRN12622000799752) Population: EPP and X-linked protoporphyria Age: ≥18 y Enrollment (estimated) N = 22 pts | Phase 2 randomized, open-label study of bitopertin to evaluate the safety, tolerability, efficacy, and protoporphyrin IX (PPIX) concentration in participants with EPP and X-linked protoporphyria (XLP) | Recruiting | ||
| AURORA (NCT05308472) Population: EPP Age: ≥18 y Enrollment N = 75 pts | Phase 2 randomized, double-blind, placebo-controlled study of bitopertin to evaluate the safety, tolerability, efficacy, and PPIX concentrations in participants with EPP | Active, not recruiting | ||
| Metabolic targets | Mitapivat (AG-348) | ENERGIZE (NCT04770753) Population: β-thal with or without α-globin gene mutations, HbE/β-thal, or α-thal/HbH disease Age: ≥18 y Enrolled N = 194 pts | Phase 3 Efficacy and safety of mitapivat in pts with α- or β-NTDT | Active, not recruiting |
| ENERGIZE-T (NCT04770779) Population: β-thal with or without α-globin gene mutations, HbE/β-thal, or α-thal/HbH disease Age: ≥18 y Enrolled N = 258 pts | Phase 3 Efficacy and safety of mitapivat in pts with α- or β-TDT | Active, not recruiting | ||
| SATISFY (NCT05935202) Population: RBC membranopathy or CDAII Age: ≥18 y Enrollment (estimated) N = 25 pts | Phase 2 Safety and efficacy of mitapivat in adult patients with membranopathies | Not yet recruiting | ||
| NCT04610866 Population: HbSS Age: 18-70 y Enrolled N = 15 pts | Phase 1/2 extension of a phase 1 pilot study of mitapivat Safety, tolerability, pharmacokinetics, and pharmacodynamics of long-term mitapivat dosing in subjects with stable SCD | Active, not recruiting | ||
| RISE UP (NCT05031780) Population: HbSS, HbSC, HbS/beta0 thal, HbS/ beta+ thal, or other SCD variants Age: ≥16 y Enrolled N = 277 pts | Phase 2/3 Double-blind, randomized, placebo-controlled, multicenter study to evaluate the efficacy and safety of mitapivat in subjects with SCD | Active, not recruiting | ||
| AG-946 | NCT04536792 Population: SCD Age:18-55 y Enrollment (estimated) N = 118 (actual) N = 122 | Phase 1 A phase 1 study for safety, tolerability, pharmacokinetics, and pharmacodynamics of AG-946 in healthy volunteers and in subjects with SCD | Completed | |
| Etavopivat (FT-4202) | HIBISCUS (NCT04624659) Population: SCD Age: 12-65 y Enrollment (estimated) N = 344 pts | Phase 2/3 An adaptive, randomized, placebo-controlled, double-blind, multicenter study of oral etavopivat, a PK activator in patients with SCD | Recruiting | |
| GLADIOLUS (NCT04987489) Population: SCD; β-thal, HbE/ β-thal or HbH (α-thal), or other thal variant Age: 12-65 y Enrollment (estimated) N = 60 pts | Phase 2 Open-label study to evaluate safety and clinical activity of etavopivat in patients with thalassemia or SCD | Recruiting | ||
| NCT05953584 Population: HbSS, HbSβ0 thal Age: 12-16 y Enrollment (estimated) N = 46 pts | Phase 2 Open-label study to evaluate the activity of etavopivat on transcranial doppler velocities in pediatric patients with SCD who are at increased risk for primary stroke | Recruiting | ||
| NCT05725902 Population: HbSS or HbS/β0 thal Age: 12-21 y Enrollment (estimated) N = 12 pts | Phase 2 Effect of etavopivat on cerebral hemodynamic response in children with SCD | Not yet recruiting | ||
| Targeting hemolysis and the vascular endothelial axis | Hpx (CSL889) | NCT04285827 Population: SCD Age: 18-60 y Enrolled N = 28 pts | Phase 1 A 2-part, phase 1, multicenter, single-dose, open-label study to evaluate the safety, tolerability, and pharmacokinetics of CSL889 in adult patients with SCD | Completed |
| l-Arginine | STArT (NCT04839354) Population: SCD (any genotype) Age: 3-21 y Enrollment (estimated) N = 360 pts | Phase 3 SCD treatment with arginine therapy (STArT) Trial | Recruiting | |
| R34 pK/PD (NCT02447874) Population: HbSS, HbSβ0 thal, Age: 7-21 y Enrollment (estimated) N = 21 pts | Phase 1/2 Arginine therapy for the treatment of VOCs in children with severe SCD | Recruiting | ||
| Complement (AP) Crovalimab | CROSSWALK-a (NCT04912869) Population: HbSS or HbSβ0 thal Age: 12-55 y Enrollment (estimated) N = 30 pts | Phase 1 A phase 1b randomized, placebo-controlled study evaluating the safety, pharmacokinetics, pharmacodynamics, and efficacy of crovalimab for the management of acute uncomplicated VOCs in patients with SCD | Recruiting | |
| CROSSWALK-c (NCT05075824) Population: HbSS or HbSβ0 thal Age:12-55 y Enrollment (estimated) N = 90 pts | Phase 2 A randomized double-blind phase 2a study evaluating the efficacy, safety, pharmacokinetics, and pharmacodynamics of crovalimab as adjunct treatment in prevention of VOCs in SCD | Recruiting | ||
| r-ADAMTS13 (SHP665) | RAISE (NCT03997760) Population: HbSS or HbSβ0 thal Age: 18-65 y Enrolled N = 9 pts | Phase 1 A study of SHP655 (rADAMTS13) in SCD | Completed | |
| ω-3 fatty acid | NCT05758766 Population: HbSS or HbSβ0 thal at steady state Age: 5-18 y Enrollment (estimated) N = 30 pts | Interventional, not applicable Study on use of plat extracts of ω-3 fatty acids to improve outcomes in individuals with SCD | Recruiting | |
| Epeleuton (DS102) | NCT05861453 Population: HbSS or HbSβ0 thal Age: ≥18 y Enrollment (estimated) N = 30 pts | Phase 2 Pharmacokinetics, pharmacodynamics, and safety of epeleuton in patients with SCD | Recruiting | |
| Inclacumab | THRIVE-131 (NCT04935879) Population: HbSS, HbSC, HbS/beta0 thal, HbS/beta+ thal Age: ≥12y Enrollment N = 232 | Phase 3 A randomized, double-blind, placebo-controlled, multicenter study to assess the safety and efficacy of inclacumab in participants with SCD experiencing VOCs | Active, not recruiting | |
| THRIVE-132 (NCT04927247) Population: SCD (any genotype) Age: ≥12y Enrollment N = 72 | Phase 3 A randomized, double-blind, placebo-controlled, multicenter study of a single dose of inclacumab to reduce re-admission in participants with SCD and recurrent VOCs | Completed | ||
| THRIVE-133 OLE (NCT05348915) Population: SCD Age: ≥12 y Enrollment (estimated) N = 520 | Phase 3 An open-label extension study to evaluate the long-term safety of inclacumab administered to participants with SCD, who have participated in an inclacumab clinical trial | Recruiting | ||
| Tocilizumab | NCT05640271 Population: HbSS, HbSC, HbS/beta0 thal, HbS/ beta+ thal Age: ≥18y Enrollment (estimated) N = 200 | Phase 2 Low-dose tocilizumab for acute chest syndrome in pts with SCD | Recruiting |
| Mechanism . | Agent . | Study title/NCT/populations . | Clinical phase . | Status . |
|---|---|---|---|---|
| Drug directly targeting erythropoiesis | Luspatercept | NCT04143724 Population: β-thal or HbE/β-thal Age: 6-17 y Enrollment (estimated) N = 54 pts | Phase 2 A phase 2a study of the safety and pharmacokinetics of luspatercept (ACE-536) in pediatric participants under regular transfusions due to (β)-thalassemia | Recruiting |
| NCT05664737 Population: α-thal HbH disease Age: ≥18 y Enrollment (estimated) N = 177 pts | Phase 2 A phase 2, double-blind, randomized, placebo-controlled, multicenter study to determine the efficacy and safety of luspatercept (BMS-986346/ACE-536) for the adults with alpha (α)-thalassemia | Recruiting | ||
| EudraCT number: 2020-005736-30 Population: CDAII (biallelic causative mutations in SEC23B gene alone or associated with other gene variants or monoallelic causative mutations in SEC23B gene in presence of hypoglycosylation of band 3 and/or associated with other gene variants) Age: ≥18 y Enrollment (estimated) N = 40 pts | Phase 2 A phase 2, multicenter, open-label study. Efficacy and safety of luspatercept (ACE-536) in adult patients with CDA II | Active, not recruiting | ||
| Targeting iron homeostasis or heme synthesis | FPN blocker | ViSionSerenity (NCT04817670) Population: HbSS or HbS/β0 thal Age:18-60 y Enrollment (estimated) N = 24 pts | Phase 2 Double-blind, randomized, placebo-controlled, efficacy, and safety study of multiple doses of VIT-2763 in subjects with SCD | Recruiting |
| Bitopertin | NCT05828108 Population: steroid-refractory DBA Age: 18-100 y Enrollment (estimated) N = 30 pts | Phase 1/2 Intrapatient dose-escalation study of the selective GlyT1 inhibitor bitopertin for steroid-refractory Diamond-Blackfan anemia | Recruiting | |
| BEACON (ACTRN12622000799752) Population: EPP and X-linked protoporphyria Age: ≥18 y Enrollment (estimated) N = 22 pts | Phase 2 randomized, open-label study of bitopertin to evaluate the safety, tolerability, efficacy, and protoporphyrin IX (PPIX) concentration in participants with EPP and X-linked protoporphyria (XLP) | Recruiting | ||
| AURORA (NCT05308472) Population: EPP Age: ≥18 y Enrollment N = 75 pts | Phase 2 randomized, double-blind, placebo-controlled study of bitopertin to evaluate the safety, tolerability, efficacy, and PPIX concentrations in participants with EPP | Active, not recruiting | ||
| Metabolic targets | Mitapivat (AG-348) | ENERGIZE (NCT04770753) Population: β-thal with or without α-globin gene mutations, HbE/β-thal, or α-thal/HbH disease Age: ≥18 y Enrolled N = 194 pts | Phase 3 Efficacy and safety of mitapivat in pts with α- or β-NTDT | Active, not recruiting |
| ENERGIZE-T (NCT04770779) Population: β-thal with or without α-globin gene mutations, HbE/β-thal, or α-thal/HbH disease Age: ≥18 y Enrolled N = 258 pts | Phase 3 Efficacy and safety of mitapivat in pts with α- or β-TDT | Active, not recruiting | ||
| SATISFY (NCT05935202) Population: RBC membranopathy or CDAII Age: ≥18 y Enrollment (estimated) N = 25 pts | Phase 2 Safety and efficacy of mitapivat in adult patients with membranopathies | Not yet recruiting | ||
| NCT04610866 Population: HbSS Age: 18-70 y Enrolled N = 15 pts | Phase 1/2 extension of a phase 1 pilot study of mitapivat Safety, tolerability, pharmacokinetics, and pharmacodynamics of long-term mitapivat dosing in subjects with stable SCD | Active, not recruiting | ||
| RISE UP (NCT05031780) Population: HbSS, HbSC, HbS/beta0 thal, HbS/ beta+ thal, or other SCD variants Age: ≥16 y Enrolled N = 277 pts | Phase 2/3 Double-blind, randomized, placebo-controlled, multicenter study to evaluate the efficacy and safety of mitapivat in subjects with SCD | Active, not recruiting | ||
| AG-946 | NCT04536792 Population: SCD Age:18-55 y Enrollment (estimated) N = 118 (actual) N = 122 | Phase 1 A phase 1 study for safety, tolerability, pharmacokinetics, and pharmacodynamics of AG-946 in healthy volunteers and in subjects with SCD | Completed | |
| Etavopivat (FT-4202) | HIBISCUS (NCT04624659) Population: SCD Age: 12-65 y Enrollment (estimated) N = 344 pts | Phase 2/3 An adaptive, randomized, placebo-controlled, double-blind, multicenter study of oral etavopivat, a PK activator in patients with SCD | Recruiting | |
| GLADIOLUS (NCT04987489) Population: SCD; β-thal, HbE/ β-thal or HbH (α-thal), or other thal variant Age: 12-65 y Enrollment (estimated) N = 60 pts | Phase 2 Open-label study to evaluate safety and clinical activity of etavopivat in patients with thalassemia or SCD | Recruiting | ||
| NCT05953584 Population: HbSS, HbSβ0 thal Age: 12-16 y Enrollment (estimated) N = 46 pts | Phase 2 Open-label study to evaluate the activity of etavopivat on transcranial doppler velocities in pediatric patients with SCD who are at increased risk for primary stroke | Recruiting | ||
| NCT05725902 Population: HbSS or HbS/β0 thal Age: 12-21 y Enrollment (estimated) N = 12 pts | Phase 2 Effect of etavopivat on cerebral hemodynamic response in children with SCD | Not yet recruiting | ||
| Targeting hemolysis and the vascular endothelial axis | Hpx (CSL889) | NCT04285827 Population: SCD Age: 18-60 y Enrolled N = 28 pts | Phase 1 A 2-part, phase 1, multicenter, single-dose, open-label study to evaluate the safety, tolerability, and pharmacokinetics of CSL889 in adult patients with SCD | Completed |
| l-Arginine | STArT (NCT04839354) Population: SCD (any genotype) Age: 3-21 y Enrollment (estimated) N = 360 pts | Phase 3 SCD treatment with arginine therapy (STArT) Trial | Recruiting | |
| R34 pK/PD (NCT02447874) Population: HbSS, HbSβ0 thal, Age: 7-21 y Enrollment (estimated) N = 21 pts | Phase 1/2 Arginine therapy for the treatment of VOCs in children with severe SCD | Recruiting | ||
| Complement (AP) Crovalimab | CROSSWALK-a (NCT04912869) Population: HbSS or HbSβ0 thal Age: 12-55 y Enrollment (estimated) N = 30 pts | Phase 1 A phase 1b randomized, placebo-controlled study evaluating the safety, pharmacokinetics, pharmacodynamics, and efficacy of crovalimab for the management of acute uncomplicated VOCs in patients with SCD | Recruiting | |
| CROSSWALK-c (NCT05075824) Population: HbSS or HbSβ0 thal Age:12-55 y Enrollment (estimated) N = 90 pts | Phase 2 A randomized double-blind phase 2a study evaluating the efficacy, safety, pharmacokinetics, and pharmacodynamics of crovalimab as adjunct treatment in prevention of VOCs in SCD | Recruiting | ||
| r-ADAMTS13 (SHP665) | RAISE (NCT03997760) Population: HbSS or HbSβ0 thal Age: 18-65 y Enrolled N = 9 pts | Phase 1 A study of SHP655 (rADAMTS13) in SCD | Completed | |
| ω-3 fatty acid | NCT05758766 Population: HbSS or HbSβ0 thal at steady state Age: 5-18 y Enrollment (estimated) N = 30 pts | Interventional, not applicable Study on use of plat extracts of ω-3 fatty acids to improve outcomes in individuals with SCD | Recruiting | |
| Epeleuton (DS102) | NCT05861453 Population: HbSS or HbSβ0 thal Age: ≥18 y Enrollment (estimated) N = 30 pts | Phase 2 Pharmacokinetics, pharmacodynamics, and safety of epeleuton in patients with SCD | Recruiting | |
| Inclacumab | THRIVE-131 (NCT04935879) Population: HbSS, HbSC, HbS/beta0 thal, HbS/beta+ thal Age: ≥12y Enrollment N = 232 | Phase 3 A randomized, double-blind, placebo-controlled, multicenter study to assess the safety and efficacy of inclacumab in participants with SCD experiencing VOCs | Active, not recruiting | |
| THRIVE-132 (NCT04927247) Population: SCD (any genotype) Age: ≥12y Enrollment N = 72 | Phase 3 A randomized, double-blind, placebo-controlled, multicenter study of a single dose of inclacumab to reduce re-admission in participants with SCD and recurrent VOCs | Completed | ||
| THRIVE-133 OLE (NCT05348915) Population: SCD Age: ≥12 y Enrollment (estimated) N = 520 | Phase 3 An open-label extension study to evaluate the long-term safety of inclacumab administered to participants with SCD, who have participated in an inclacumab clinical trial | Recruiting | ||
| Tocilizumab | NCT05640271 Population: HbSS, HbSC, HbS/beta0 thal, HbS/ beta+ thal Age: ≥18y Enrollment (estimated) N = 200 | Phase 2 Low-dose tocilizumab for acute chest syndrome in pts with SCD | Recruiting |
AP, alternative pathway; HbE, hemoglobin E; HbH, hemoglobin H; HbS, hemoglobin S; HbSS, homozygous hemoglobin S; HbSC, hemoglobin SC; NCT, National Clinical Trial; pts, patients; RBC, red blood cells; thal, thalassemia; TD, transfusion dependent.
∗Studies outcomes and dosages are reported in supplemental Table 2.
Molecules targeting iron homeostasis or heme synthesis
In erythropoiesis, iron is crucial for hemoglobinization but cytotoxic as free iron. In red cell disorders such as thalassemias or SCD, free iron negatively affect erythropoiesis, red cell features, and survival in the peripheral circulation (Figures 1-2).21-24 Thus, modulation of iron homeostasis or heme biosynthesis might represent an interesting approach to improve anemia in hereditary red cell disorders.
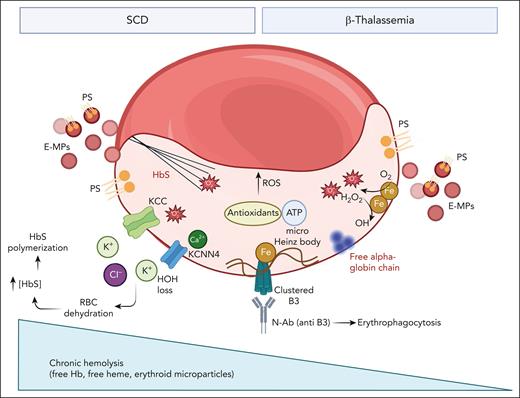
Effects of free iron on red cells from patients with either β-thalassemia or SCD. In pathologic red cells free iron sustains chronic oxidation with generation of reactive oxygen species (ROS) throughout the Fenton reaction. This requires an efficient antioxidant machinery with the metabolic support of ATP (see also Figure 3). The chronic and severe red cell membrane damage is further amplified respectively by membrane association of free alpha chains in β-thalassemic erythrocytes and cyclic polymerization/depolymerization events in sickle red cells. In both disorders, red cell membrane oxidation results in (1) increased membrane mechanical instability favoring to abnormally clusterization of oxidized band 3 (B3); (2) exposition of phosphatidyl serine (PS); and (3) generation of erythroid microparticles (E-MP), also carrying PS. The cumulative effects of oxidation are the premature red cell aging with accelerated removal by erythrophagocytosis mediated by both PS exposure and naturally occurring anti–band 3 antibody (N-Ab). In addition, in sickle erythrocytes, membrane damage is associated with increase permeability to Ca2+ with the activation of the Gardos channel (KCNN4) coupled with the oxidation induced activation of the K-Cl (KCC) cotransport. This ends in sickle red cell dehydration, relative increase in HbS concentration with a negative impact on HbS polymerization kinetic. Of note, in SCD a smaller component of hemolysis takes place intravascularly with saturation of physiologic binding proteins (eg, hemopexin) allowing for the presence of free heme and Hb in the peripheral circulation.
Effects of free iron on red cells from patients with either β-thalassemia or SCD. In pathologic red cells free iron sustains chronic oxidation with generation of reactive oxygen species (ROS) throughout the Fenton reaction. This requires an efficient antioxidant machinery with the metabolic support of ATP (see also Figure 3). The chronic and severe red cell membrane damage is further amplified respectively by membrane association of free alpha chains in β-thalassemic erythrocytes and cyclic polymerization/depolymerization events in sickle red cells. In both disorders, red cell membrane oxidation results in (1) increased membrane mechanical instability favoring to abnormally clusterization of oxidized band 3 (B3); (2) exposition of phosphatidyl serine (PS); and (3) generation of erythroid microparticles (E-MP), also carrying PS. The cumulative effects of oxidation are the premature red cell aging with accelerated removal by erythrophagocytosis mediated by both PS exposure and naturally occurring anti–band 3 antibody (N-Ab). In addition, in sickle erythrocytes, membrane damage is associated with increase permeability to Ca2+ with the activation of the Gardos channel (KCNN4) coupled with the oxidation induced activation of the K-Cl (KCC) cotransport. This ends in sickle red cell dehydration, relative increase in HbS concentration with a negative impact on HbS polymerization kinetic. Of note, in SCD a smaller component of hemolysis takes place intravascularly with saturation of physiologic binding proteins (eg, hemopexin) allowing for the presence of free heme and Hb in the peripheral circulation.
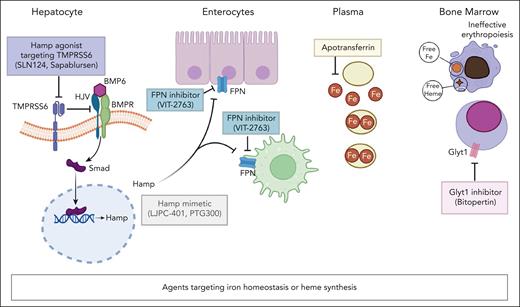
Therapeutic strategies targeting iron homeostasis or heme synthesis. Hamp agonist(s) block TMPRSS6 function (SLN124, sapablursen), resulting in Smad phosphorylation and nuclear translocation. This ends in upregulation of Hamp expression. Hamp mimetics (LJPC-401, PTG300) increase Hamp level. Hamp inhibits the iron export activity of FPN in enterocytes and macrophages. FPN blocker(s) (VIT-2763), mimic Hamp function. Apotransferrin binds circulating free iron (Fe2+). Collectively, these strategies targeting iron homeostasis have been proposed to reduce free iron to rebalance iron/heme synthesis and globin synthesis in β-thalassemia, which is characterized by ineffective erythropoiesis. Bitopertin as Glyt1 inhibitor blocks the import of glycine, which is the first step in the metabolic cascade of heme biosynthesis. Bitopertin treatment has been shown to reduce PPIX erythroblast content. BMP6, bone morphogenic protein 6; BMPR, bone morphogenic protein-receptor; HJV, hemojuvelin; Smad, suppressor of mothers against decapentaplegic; TMPRSS6, transmembrane protease serine 6.
Therapeutic strategies targeting iron homeostasis or heme synthesis. Hamp agonist(s) block TMPRSS6 function (SLN124, sapablursen), resulting in Smad phosphorylation and nuclear translocation. This ends in upregulation of Hamp expression. Hamp mimetics (LJPC-401, PTG300) increase Hamp level. Hamp inhibits the iron export activity of FPN in enterocytes and macrophages. FPN blocker(s) (VIT-2763), mimic Hamp function. Apotransferrin binds circulating free iron (Fe2+). Collectively, these strategies targeting iron homeostasis have been proposed to reduce free iron to rebalance iron/heme synthesis and globin synthesis in β-thalassemia, which is characterized by ineffective erythropoiesis. Bitopertin as Glyt1 inhibitor blocks the import of glycine, which is the first step in the metabolic cascade of heme biosynthesis. Bitopertin treatment has been shown to reduce PPIX erythroblast content. BMP6, bone morphogenic protein 6; BMPR, bone morphogenic protein-receptor; HJV, hemojuvelin; Smad, suppressor of mothers against decapentaplegic; TMPRSS6, transmembrane protease serine 6.
In the last 2 decades, a much-expanded knowledge of iron homeostasis has resulted in the identification of multiple functional targets.25-27 Hepcidin (Hamp) and ferroportin (FPN) play an essential role in regulating iron absorption and iron organ accumulation. In the presence of ineffective erythropoiesis, erythroblasts release excessive amounts of erythroferrone, which acts as Hamp suppressor. Reduced Hamp affects the duodenal FPN externalization, establishing an iron overabsorption vicious cycle that sustains organ iron accumulation (Figure 2).28 Studies in animal models of β-thalassemia show amelioration of anemia and ineffective erythropoiesis as well as improvement of organ iron overload when treated with (1) Hamp-mimetic; (2) Hamp agonist such as vamifeport (VIT-2793), an oral FPN inhibitor, or the transmembrane protease serine 6 ligand conjugated antisense agent; and (3) apotransferrin (Figure 2).29-32 However, clinical studies on iron restriction in patients with TDT or NTDT were either unsuccessful or terminated early (supplemental Table 3).33,34
Bitopertin, a glycine transport inhibitor that reduces heme synthesis (Figure 2) had a similar fate. Bitopertin was expected to rebalance heme and globin synthesis and to reduce erythroid oxidation.35,36 Although Bitopertin improved ineffective erythropoiesis and red cell survival in β-thalassemic mice, the clinical study in patients with NTDT (NCT03271541) was discontinued due to the lack of efficacy as determined by an interim analysis (supplemental Table 3).34 Diamond-Blackfan anemia (DBA) and erythropoietic protoporphyria (EPP) seem to offer new possibilities for bitopertin. In the first case, the rationale is represented by the accumulation of free heme that overcomes the expression of cyto-protective systems in cell models and ex vivo in CD34+ derived cells from patients with DBA.37 A phase 1 to 2 intrapatient dose-escalation study of bitopertin for steroid-refractory DBA (NCT 05828108) has been recently activated (Table 1; supplemental Table 2). In EPP, in vitro evidence shows improvement of EPP cell metabolism and reduced protoporphyrin IX synthesis in the presence of bitopertin.38 This generated the rationale to design the open-label clinical trial BEACON (ACTRN12622000799752) and the phase 2 AURORA double-blind placebo-controlled trial (NCT05308472) in adult patients with EPP and X-linked porphyria (Table 1; supplemental Table 2). Encouraging ad interim data come from both studies, showing dose-dependent and sustained reductions of plasma protoporphyrin IX levels, amelioration of sunlight tolerance, stable Hb, and grade 1 to 2 adverse events (AEs; eg, limited dizziness, lightheadedness, headache, and nausea). In AURORA study, a significant improvement in the patient global impression of change was also reported in patient with EPP treated with bitopertin 60 mg once daily, compared with placebo group. Considering the heterogeneous group of porphyrias, givosiran, a small interfering RNA reducing the activity of liver aminolevulinic acid–synthase-1, has been recently approved by the FDA and EMA for the treatment of acute hepatic porphyrias.39,40
Altogether, the failures of molecules targeting iron homeostasis or heme synthesis in β-thalassemia when translated from preclinical to clinical studies can be explained by functional differences in iron homeostasis between human and murine β-thalassemic erythropoiesis. Multiple factors, in addition to free iron, determine the severity of oxidation in β-thalassemic cells, which ultimately overwhelms adaptive mechanisms such as autophagy and drives β-thalassemic cells toward energy depletion and apoptosis.36,41,42 These agents could be successful in disorders of erythropoiesis with preserved maturation profile such as polycythemia vera.43-46
Finally, the modulation of iron homeostasis as therapeutic option has been also explored in patients with SCD.47-51 In SCD, iron restriction decreases hemoglobin S (HbS) synthesis, resulting in reduction of the cellular HbS concentration, which in turn modulates the kinetic of HbS polymerization with a secondary beneficial impact on red cell oxidative stress (Figure 1).47-50 In observational studies in patients with SCD, iron restriction obtained by either phlebotomy or erythropheresis resulted in a marked reduction of pain crisis rate and an improvement of patient’s quality of life.47-50 Recently, humanized mouse model for SCD exposed to iron restriction by either diet or vamifeport (VIT-2793, an oral FPN inhibitor) showed an improvement of sickle red cell features, a reduction of sickling, with a beneficial effect on inflammatory vasculopathy.52,53 These data further support the rationale for ViSionSerenity (NCT04817670), a phase 2a, double-blind, randomized, placebo-controlled study to evaluate the efficacy and safety of multiple doses of vamifeport in patients with SCD (Table 1; supplemental Table 2).
Agents targeting erythroid metabolism
The long life span of erythrocytes into the peripheral circulation requires efficient metabolic and antioxidant machinery. In red cells, the metabolic keystone is adenosine triphosphate (ATP), which is produced by the glycolytic degradative process, involving pyruvate kinase (PK) in the final step. 2-3 diphosphoglycerate (2,3-DPG) is an intermediate metabolite of the glycolytic pathway (Figure 3). The 2,3-DPG/ATP ratio is critical to ensure normal functioning and survival of red cells into the peripheral circulation.6,54 In addition, the existing crossconnection between glycolysis and the pentose phosphate shunt crucially contributes to the production of NAD phosphate and reduced nicotinamide adenine dinucleotide (NADH) (Figures 2-3). Of note, a substantial portion of key antioxidant systems, such as glutathione (GSH), are NAD or NAD phosphate dependent.6,55-57 In SCD or β-thalassemia, despite enhanced glycolysis in response to increased NADH demand, antioxidant systems may not be able to cope with severe and persistent oxidative stress.6,55-57 This might also worsen the red cell membrane mechanical instability, contributing to the decrease life span of pathologic erythrocytes. The situation becomes more complex in hereditary spherocytosis (HS), which is characterized by reduced red cell deformability due to defects in membrane protein(s) and a relative PK deficiency.58-61
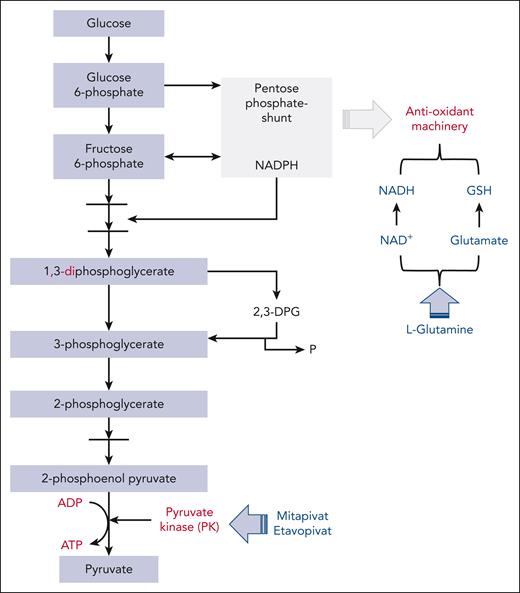
Pathways targeted by PK(s) activators and l-glutamine in red cells. The glycolytic pathway generates ATP and interfaces the pentose phosphate shunt, which is the main source of reduced nicotinamide adenine dinucleotide phosphate (NADPH). This is required by antioxidant systems and the Rapoport-Luebering shunt that generates 2,3-DPG. PK is the last enzyme in the glycolytic pathway. In SCD, the intense and sustain oxidation results in consumption of NADH and glutathione, favoring hemolysis. l-Glutamine as glutamate might support NADH and GSH systems, reducing red cell oxidation. ADP, adenosine diphosphate; GSH, glutathione; NADP, NAD phosphate.
Pathways targeted by PK(s) activators and l-glutamine in red cells. The glycolytic pathway generates ATP and interfaces the pentose phosphate shunt, which is the main source of reduced nicotinamide adenine dinucleotide phosphate (NADPH). This is required by antioxidant systems and the Rapoport-Luebering shunt that generates 2,3-DPG. PK is the last enzyme in the glycolytic pathway. In SCD, the intense and sustain oxidation results in consumption of NADH and glutathione, favoring hemolysis. l-Glutamine as glutamate might support NADH and GSH systems, reducing red cell oxidation. ADP, adenosine diphosphate; GSH, glutathione; NADP, NAD phosphate.
The development of PK activators has changed the scenario of the treatment of patients with PK deficiency, but also of other red cell disorders characterized by either severe oxidation as in thalassemias and SCD (Figure 1) or relative PK deficiency as in HS.54,62,63 Three oral drugs targeting PK function have been recently evaluated: (1) mitapivat and AG-946, which bind the PK enzyme pocket at the dimer-dimer interface, resulting in PK activation of both PK-R and PK-M2; and (2) etavopivat, a selective oral PK-R activator.54 Mitapivat has been approved by the FDA and the EMA for the treatment of hemolytic anemia in adult patients with PK deficiency (supplemental Table 4).64,65
Preclinical studies in a mouse model for β-thalassemia have shown the beneficial effects of mitapivat on both ineffective erythropoiesis and chronic hemolytic anemia with an accompanying improvement of iron homeostasis.66,67 In a phase 2 proof-of-concept study (NCT03692052), the effects of mitapivat were evaluated in a cohort of patients with NTDT, including both α- and β-thalassemic genotypes (supplemental Table 1).68,69 Mitapivat improved anemia in 80% of patients with NTDT within the first 6 weeks of treatment (sustained increase of Hb >1.0 g/dL) and reduced markers of ineffective erythropoiesis and hemolysis.69 Of note, all patients with α-thal responded to mitapivat treatment. The AEs were grade 1 to 2 (eg, insomnia, dizziness, or headache), similar to those reported in patients with PK deficiency treated with mitapivat.69 Two phase 3, multicenter, randomized, double-blind, placebo-controlled trials to assess the efficacy and safety of mitapivat in adults with α- or β-NTDT (ENERGIZE [NCT04770753]) or TDT (ENERGIZE-T [NCT04770779]) are ongoing and have recently completed patient enrollment (Table 1; supplemental Table 2).
The beneficial effects of mitapivat on ineffective erythropoiesis in β-thalassemia has led to the consideration of mitapivat in other hereditary red cell disorders associated with perturbation of erythropoiesis, such as CDAII.70-72 An European/Canadian prospective, multicenter, single-arm phase 2 trial (SATISFY [NCT05935202]) on patients with CDAII is expected to start enrollment in 2024 (Table 1; supplemental Table 2). Of note, SATISFY also involves membranopathies, such as HS, based on (1) abnormalities in metabolome of HS red cell58-61; and (2) the improvement of anemia in a mouse model for mild HS.73
In SCD, the abnormally increased 2,3-DPG and the reduced ATP are powerful determinants of HbS deoxygenation/sickling, with a negative impact also on responsiveness to oxidant damage related to reduced energy availability.74,75 Both mitapivat and etavopivat have then been tested in mouse models for SCD, displaying an improvement of 2,3-DPG/ATP ratio, increased Hb oxygen affinity, and reduced sickling. This was associated with decreased markers of chronic hemolysis and amelioration of some red cell features (eg, markers of red cell oxidation, mitochondria red cell retention, and extramedullary erythropoiesis).76,77
In a proof-of-concept study (NCT04000165; supplemental Table 1), mitapivat improved anemia in patients with SCD, reducing markers of hemolysis, increasing ATP/2,3-DPG ratio and activating the Lands cycle involved in membrane lipid remodeling.78,79 A phase 2 open-label study with mitapivat in patients with SCD (ESTIMATE study; www.trial-register.nl [NL8517]; supplemental Table 1) showed a significant increased Hb (>1 g/dL in 75% of SCD patients) and a decrease of markers of hemolysis associated with the encouraging observation of reduced annualized vaso-occlusive crises (VOCs) compared with patients’ historical baseline. Mitapivat was well tolerated, and AEs were mainly grade 1 to 2 (eg, headache, increase aspartate aminotransferase, alanine aminotransferase, and dyspepsia).80 As shown in Table 1, there are 2 ongoing clinical studies with mitapivat in patients with SCD. The first is the extension study (NCT04610866) of a previous phase 1 study (NCT04000165; supplemental Table 1).78 Data for up to 2 years show that mitapivat is safe and well tolerated in patients with SCD with evidence of sustained long-term improvements in Hb, hemolytic, and sickling kinetics.81 The second is a 2/3 phase double-blind, randomized, placebo-controlled trial (RISE UP [NCT05031780]) evaluating the efficacy and safety of mitapivat in patients with SCD. Results from the phase 2 double-blind period show that treatment with mitapivat produced statistically significant and clinically meaningful improvement in Hb response at both dose levels (50 mg twice daily and 100 mg twice daily) compared with placebo, with a safety profile consistent with previous studies (Table 1; supplemental Table 2).82 Indeed, the primary end point was achieved in 46.2% of patients at 50 mg twice daily and in 50% of patients at 100 mg twice daily.82 Of note, a reduction in the annualized rate of sickle cell pain was observed in patients with SCD from both mitapivat-treated groups compared with placebo (0.83 and 0.51, respectively, in mitapivat 50 mg twice daily and 100 mg twice daily groups vs 1.71 in placebo group). Although the study was not powered for this end point, these results are extremely promising for clinical management of patients with SCD and needs to be confirmed in the phase 3 study.
A next-generation PK activator with improved metabolic profile (AG-946) compared with that of mitapivat has been developed. A phase 1 clinical trial with AG-946 in healthy volunteers and in patients with SCD independently from the genotype has been recently completed (NCT04536792; Table 1).83-85
Etavopivat has been first tested in a phase 1 clinical study (NCT03815695; supplemental Table 1) in healthy individuals and volunteers with SCD. Increased ATP and decreased 2-3 DPG contents were observed in red cells from healthy controls.86 In patients with SCD, this was associated with a sustained increase in Hb in 73% of patients with SCD treated with etavopivat (400 mg daily) and a trend toward reduction of VOCs requiring hospitalization.87 Two clinical studies on etavopivat in patients with SCD (GLADIOLUS [NCT04987489] and HIBISCUS [NCT04624659], phase 2 and phase 2/3, respectively) are open to enrollment (Table 1; supplemental Table 2). Two phase 2 pediatric studies (NCT05953584 and NCT05725902, respectively; Table 1; supplemental Table 2) will evaluate the activity of etavopivat on transcranial doppler velocities in children with SCD, who are at increased risk for primary stroke, and the effect of etavopivat on cerebral hemodynamic response in children with SCD.
In SCD, another therapeutic approach to sustain the antioxidant machinery is represented by oral supplementation with l-glutamine. In red cells, the l-glutamine conversion into glutamate supports the GSH antioxidant system and the production of nicotinamide adenine dinucleotide plus by nicotinamide adenine dinucleotide synthase (Figure 3). l-Glutamine supplementation has been shown to beneficially affect sickle cell–related VOC pain rate, resulting in FDA approval for patients with SCD.88 However, the lack of pharmacodynamic/pharmacokinetic data and the uncertainties about mechanisms of action require additional studies to better understand the indications for l-glutamine in SCD.89,90 EMA did not share the positive view of FDA and rejected the application for l-glutamine in SCD, expressing concerns on the methodology used in the clinical study.
Molecules targeting Hb are still on track in SCD
Voxelotor is the only approved oral antisickling therapeutic molecule that covalently binds to HbS, weakening HbS fiber contacts. In the HOPE (hemoglobin oxygen affinity modulation to inhibit HbS polymerization) study, voxelotor ameliorated chronic hemolysis in the absence of a significant impact on the rate of VOCs in patients with SCD. FDA-approved voxelotor for adults and children aged ≥4 years with SCD, whereas EMA approved it for patients with SCD aged ≥12 years.91 The open-label extension of the HOPE study (HOPE-OLE) and the PROSPECT registry will help to determine whether voxelotor might prevent sickle cell–related organ complications.92,93 A phase 2 to 3 study on second-generation voxelotor-like antisickling agent GBT021601 in patients with SCD is now enrolling (supplemental Table 5). The ad interim data support the tolerability and the safety of GBT021601 in patients with SCD. This was associated with an improvement of anemia (2.7 g/dL Hb increase in 100 mg per day group and 3.17 g/dL in the 150 mg per day group), a reduction in adherent cells in flow adhesion assay in presence of vascular cell adhesion molecule-1, without major change in the pain rate.94
Agents targeting the hemolysis-vascular endothelial axis
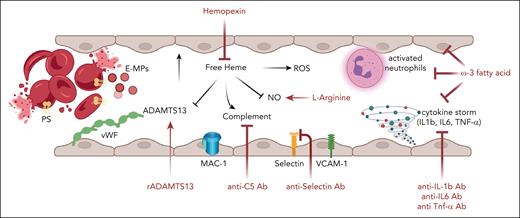
Chronic hemolysis is a distinguishing feature of thalassemias, SCD, and hereditary red cells disorders such as HS or PK deficiency. Although erythrophagocytosis plays a key role in the early removal of pathologic red cells, a smaller component of hemolysis takes place intravascularly. This might be further increased by splenectomy.95-98 Consumption of hemopexin (Hpx), the physiologic buffer of free heme, increases plasma-free heme. In SCD, the detrimental effect of free heme results in (1) local nitric oxide (NO) deficiency and plasma pro-oxidant environment; (2) activation of complement system; (3) inhibition of ADAMTS13 activity, with relative ADAMTS13 deficiency and accumulation of large multimers of von Willebrand factor (Figure 4). Altogether, these changes contribute to chronic inflammatory vasculopathy, resulting in upregulation of proinflammatory cytokines and markers of vascular endothelial activation, such as vascular cell adhesion molecule-1 or selectin (Figure 4).99-102
Agents targeting the hemolysis-vascular endothelial axis. In SCD, one-third of the chronic hemolysis happens to be intravascular. This is also associated with the release of erythroid microparticles, which also contains heme. Thus, the consumption of physiologic binding proteins respectively haptoglobin for hemoglobin and Hpx for heme, results increased level of free hemoglobin and free heme into the peripheral circulation, promoting a plasmatic pro-oxidant environment. This contributes to chronic, unresolved inflammation characterizing SCD resulting in the upregulation of proinflammatory cytokines (Il-1β, IL-6, and TNF-α) and makers of vascular endothelial activation such as vascular cell adhesion molecule-1 (VCAM-1) or selectin. To counteract the detriment effects of free heme and to limit inflammatory vasculopathy, the following novel therapeutic strategies are under evaluation in clinical trials in patients with SCD (Table 1): (1) recombinant ADAMTS13 (r-ADAMTS13) for the relative ADAMTS13 functional deficiency; (2) crovalimab as anti-C5 inhibitor to block the overactivation of the complement alternative pathway; (3) l-Arginine to support NO synthesis; (4) anti–P-selectin antibodies to prevent cell-cell adhesion between red cells and neutrophils to vascular endothelial cells; (5) blockers of the proinflammatory cytokines (anti–IL-1b, –IL-6, or –TNF-α antibodies); and (6) multitarget approach by ω-3 fatty acid supplementation. IL-1, interlukin-1; TNF-α, tumor necrosis factor α.
Agents targeting the hemolysis-vascular endothelial axis. In SCD, one-third of the chronic hemolysis happens to be intravascular. This is also associated with the release of erythroid microparticles, which also contains heme. Thus, the consumption of physiologic binding proteins respectively haptoglobin for hemoglobin and Hpx for heme, results increased level of free hemoglobin and free heme into the peripheral circulation, promoting a plasmatic pro-oxidant environment. This contributes to chronic, unresolved inflammation characterizing SCD resulting in the upregulation of proinflammatory cytokines (Il-1β, IL-6, and TNF-α) and makers of vascular endothelial activation such as vascular cell adhesion molecule-1 (VCAM-1) or selectin. To counteract the detriment effects of free heme and to limit inflammatory vasculopathy, the following novel therapeutic strategies are under evaluation in clinical trials in patients with SCD (Table 1): (1) recombinant ADAMTS13 (r-ADAMTS13) for the relative ADAMTS13 functional deficiency; (2) crovalimab as anti-C5 inhibitor to block the overactivation of the complement alternative pathway; (3) l-Arginine to support NO synthesis; (4) anti–P-selectin antibodies to prevent cell-cell adhesion between red cells and neutrophils to vascular endothelial cells; (5) blockers of the proinflammatory cytokines (anti–IL-1b, –IL-6, or –TNF-α antibodies); and (6) multitarget approach by ω-3 fatty acid supplementation. IL-1, interlukin-1; TNF-α, tumor necrosis factor α.
Hpx has been explored as candidate therapeutic option for hemolytic anemias in preclinical studies in mouse models for SCD.103-106 A phase 1 clinical study (NCT04285827) has been designed to evaluate the safety and tolerability of single dose of recombinant Hpx (CSL889) in patients with SCD without or with active VOCs. Ad interim results indicate no serious AEs related to CSL889 (Table 1; supplemental Table 2).107
An attempt to target the free heme related reduced nitric oxide (NO) bioavailability and to improve the arginine deficiency syndrome during acute events in SCD, is represented by the acute administration of l-arginine, a precursor of NO synthesis.108,109 Despite many published studies, there is still uncertainty on the role of arginine therapy in SCD. Two new ongoing studies on l-arginine supplementation may provide some much-needed clarity (Table 1; supplemental Table 2). STArT (NCT04839354) is a double-blind, placebo-controlled, randomized, phase 3, multicenter trial on the efficacy of IV l-arginine administration during VOCs in children, adolescents, and young adults with SCD. R34 pK/PD (NCT02447874), a phase 1/2 study, evaluates the pharmacokinetics of l-arginine infusion during acute VOCs in children, adolescents, and young adults with SCD as well as changes in NO metabolites.
Growing evidence indicates that overactivation of complement plays a role in sickle cell–related acute and chronic clinical manifestations. Indeed, free heme, dense red cells, erythroid microparticles, and heme induced inhibitory effect on factor I, a complement regulatory protein,110-113 synergize toward complement activation in patients with SCD.110,111,114 In preclinical studies, anti-C5 antibody or a 14E1-mouse–specific properdin inhibitor were shown to protect against acute sickle cell–related clinical manifestations, generating the rational for designing clinical trials with complement inhibitors in patients with SCD.111 Up to now, 3 clinical studies on the effects of complement inhibitors have been recruiting (Table 1; supplemental Table 2). The CROSSWALK-a (NCT04912869; phase 1) and CROSSWALK-c (NCT05075824; phase 2) trials evaluate the effects of crovalimab, an anti C5-antibody, respectively, on safety and efficacy vs placebo of IV administration in adult patients with SCD (Table 1). Another strategy to control complement overactivation in SCD is based on the block of properdin, a positive regulator of the complement system. The clinical trial Phoenix (NCT05565092) evaluates the safety of ALXN1820, a humanized bispecific variable heavy domain of heavy chain antibody that simultaneously binds albumin and properdin in patients with SCD. Although the therapeutic strategy seemed promising, this latter clinical trial has been recently suspended due to changes in company development strategies.
Considering the intense cross talk between free heme and vascular endothelial compartment, evidence in cell- and animal-based studies supports the protective effects of recombinant ADAMTS13 (rADAMTS13) against acute sickle cell–related hemolysis and organ damage.115,116 A phase 1, randomized, double-blind, placebo-controlled, multicenter, ascending, single-dose study with rADAMTS13 in patients with SCD has been designed (NCT03997760; Table 1; supplemental Table 2). No rADAMTS13-related serious treatment-emergent AEs were recorded, supporting the design of future studies on patients with SCD in acute setting.117
An holistic approach to treat inflammatory vasculopathy and unresolved inflammation has been proposed in diseases other than SCD, such as in cardiovascular disease or atherosclerosis, by administrating ω-3 fatty acids (ω-3 polyunsaturated fatty acids [PUFAs]).118-120 Previous studies in both mouse models and patients with SCD have shown beneficial effects of dietary supplementation with ω-3 PUFA preparations.121-124 As shown in Table 1, 2 clinical studies with ω-3 fatty acid supplementation are actively recruiting patients with SCD. The first one (NCT05758766) is an interventional randomized crossover trial, which studies the impact of ω-3 fatty acid supplementation derived from plant (flaxseed) on both acute and chronic pain rate and on patients’ quality of life. The second has been designed as phase 2 open-label study (NCT05861453), which evaluates epeleuton, a second-generation synthetic ω-3 fatty acid on adult patients with SCD (Table 1; supplemental Table 2).125 Epeleuton shows an advantageous functional profile compared with other formulations of ω-3 fatty acids tested in SCD.121-124 A phase 2, randomized, double-blind, placebo-controlled, parallel-group, dose-finding study showed that the docosahexaenoic acid ethyl ester SC411 increased Hb, improved markers of inflammatory vasculopathy, and reduced the use of analgesic at home and the number of days of school absence related to sickle cell pain.124 There is an open-label extension of SC411 in children with SCD (NCT02973360; supplemental Table 6).
Other therapeutic strategies to control or limit sickle cell–related inflammatory vasculopathy are represented by the P-selectin blockers, crizanlizumab or inclacumab.62 Crizanlizumab, successfully reduced VOCs in patients with SCD in the SUSTAIN study, with rates of treatment-emergent AEs similar between treatment arms across all subgroups.62 However, it was not superior to placebo in modifying the pain crisis rate in adult patients with SCD in the STAND phase 3 study (NCT03814746).126 This induced EMA to recommend the suspension of marketing authorization for crizanlizumab as a treatment of patients with SCD (https://www.ema.europa.eu/en/medicines/human/referrals/adakveo). As shown in supplemental Table 5, additional clinical studies on crizanlizumab in special SCD settings are still ongoing. Among them, primary analyses from the SPARTAN trial (NCT03938454) on SCD-related priapism show that patients treated with crizanlizumab over 26 weeks exhibited approximately half as many priapic events compared with baseline, with safety profile similar to that reported in registration trials (supplemental Table 5).127 Promising in vitro, ex vivo data on inclacumab, an anti–P-selectin immunoglobulin G4 antibody, support the ongoing phase 3 clinical trial in adult patients with SCD (THRIVE-131 [NCT04935879]; THRIVE-132 [NCT04927247]; THRIVE-133 OLE[NCT05348915]) (Table 1; supplemental Table 2).128,129
The attempt to interfere with inflammasome has been explored in a phase 2 study with canakinumab, an antibody against interleukin-1, in children-young adult patients with SCD (supplemental Table 1). Canakinumab improved the markers of chronic inflammation and patients’ fatigue but failed to reduce VOC rate.130 Up to now, tocilizumab, an anti–interleukin-6 antibody, is under evaluation in a phase 2 study (NCT05640271) in adult patients with SCD admitted to the emergency department for acute chest syndrome. Tocilizumab is expected to beneficially affect both inflammatory response and pain during acute chest syndrome (Table 1; supplemental Table 2).
Conclusions
The therapeutic portfolio for thalassemias, SCD, and other red cell disorders is constantly developing, considering a new scenario in which single agents target common physiologies in different red cell disorders. This is extremely interesting in the context of rare diseases, because it increases the size of the population using the same compounds and makes these orphan disorders more attractive for pharma investments.131
The increase in life expectancy of patients with hereditary red cell disorders might prompt the consideration of therapeutic strategies targeting inflammatory vasculopathy also in disorders other than SCD, such as NTDT or HS, in older patients, based on the synergy between hemolysis–related inflammatory vasculopathy96 and aging. Finally, we are seeing some light at the end of the tunnel when we consider luspatercept and mitapivat in clinical management of 2 orphan conditions such as α-thalassemia and CDAII, whose treatment is still based on blood transfusion and iron-chelation therapy. Collectively, these new therapeutic approaches should be considered not only as single agent but possibly in combination between them or with already standard-of-care treatments such as hydroxycarbamide in SCD.
Acknowledgments
The authors thank Carlo Brugnara, Department of Laboratory Medicine, Boston Children’s Hospital, Harvard Medical School, Boston, MA, for the fruitful discussion.
This study was supported by Ministero Italiano Ricerca Universita', Progetti di Interesse Nazionale PRIN2020 (grant 2020Z22PM7; L.D.F.).
Authorship
Contribution: V.M.P. and L.D.F. analyzed the studies and wrote the manuscript; and F.M. contributed to the literature analysis.
Conflict-of-interest disclosure: V.M.P. reports consultant fees from bluebird bio; advisory board fees from bluebird bio, Bristol Myers Squibb, and Vertex; and speakers bureau fee from Novartis. L.D.F. reports research grants from Agios and Bristol and advisory board fees from Roche. F.M. declares no competing financial interests.
Correspondence: Lucia De Franceschi, Department of Engineering for Innovative Medicine, University of Verona and AOUI Verona, P.le L. Scuro, 10, 37134 Verona, Italy; email: lucia.defranceschi@univr.it.
References
Author notes
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal