In this issue of Blood, Borate et al, on behalf of the International Consortium for Myelodysplastic Syndromes (icMDS), has authored a position paper addressing trial eligibility for patients with myelodysplastic syndrome (MDS).1 The publication emphasizes the impact of excessive exclusion criteria in these trials, highlighting the barriers they create to therapeutic progress. Although not unique to MDS-focused trials, this issue is particularly problematic for MDS.
The authors conducted a comprehensive review of inclusion and exclusion criteria in 191 clinical trials on MDS, most of which focused on high-risk MDS. Notably, despite the analysis covering the period between 2000 and 2023, none of the trials considered the International Prognostic Scoring System - Molecular (IPSS-M),2 which incorporates molecular profiling of MDS that was published in 2022 and presented at an American Society of Hematology meeting the prior year, in its inclusion criteria. This underscores a persistent lag in incorporating advances in diagnostic and prognostic tools, even within clinical trials.
Another striking finding is that exclusion criteria in most of the analyzed trials were often unrelated to drug safety signals, even in late-phase studies. This was particularly evident for common laboratory parameters such as renal and hepatic function. Given that MDS primarily affects elderly patients who often have comorbidities, excluding such patients on criteria unrelated to potential drug toxicity is highly questionable. However, it is also important to acknowledge that exclusion criteria are designed to avoid the inclusion of patients with preexisting conditions that might make attribution of an adverse event to the investigational drug more difficult.
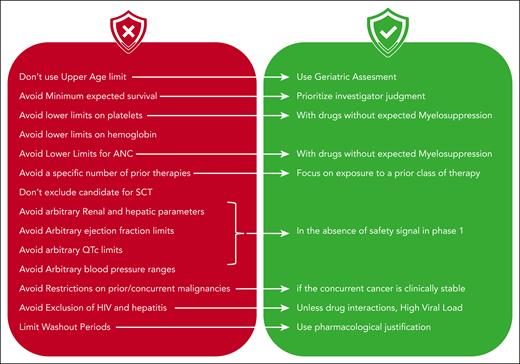
This is not a new problem (it has been previously raised3,4), but until now, no study has comprehensively analyzed eligibility criteria and their practical implications. To address this, the authors conducted a Delphi survey among icMDS experts and developed practical, straightforward guidelines for designing eligibility criteria for MDS trials. These guidelines (see figure) are extremely valuable and should be implemented in future studies.
Summary of icMDS recommendations for establishing eligibility criteria for MDS clinical trials. ANC, absolute neutrophil count; QTc, QT corrected; SCT, stem cell transplant.
Summary of icMDS recommendations for establishing eligibility criteria for MDS clinical trials. ANC, absolute neutrophil count; QTc, QT corrected; SCT, stem cell transplant.
It is crucial to acknowledge, however, that MDS trial design does face some limitations. Historical precedents in clinical trials and ambiguous regulatory requirements can impose constraints. For instance, as the authors note, if the goal of a clinical trial is to improve outcomes of higher-risk MDS patients with azacitidine, health authorities may require the study population to closely resemble that of the original azacitidine approval trial. However, conducting a trial in 2024 using eligibility criteria from a study published over 15 years ago is questionable, especially in light of the significant advancements in understanding the molecular basis of MDS since then.5 However, any modification to the study population also alters the expected outcomes in the control arm, making changes to these criteria a double-edged sword.
The need for this position paper is clear. Clinical trial development for MDS has been tumultuous in recent years. Although a few drugs, such as luspatercept and imetelstat, have demonstrated clinical benefit and received regulatory approval, many others, including APR246, roxadustat, pevonedistat, sabatolimab, and magrolimab, have failed to do so.6 Attributing these phase 3 failures solely to inclusion and exclusion criteria would be reductive. The challenges are multifaceted, including flawed evaluation parameters, overly ambitious end points, and suboptimal patient selection.
Although this article focuses on refining study inclusion criteria, related efforts, mainly from the same group or authors, have addressed other critical aspects of clinical trial design. These include initiatives to harmonize diagnostic criteria,7 improve response assessment methods,8 and establish more reliable end points.9 Ultimately, developing more effective drugs remains key to improve trial outcomes, especially for high-risk MDS. However, progress also depends on rethinking how clinical trials are conducted. This article is a valuable contribution to that effort.
Conflict-of-interest disclosure: L.A. has served on the advisory boards of Bristol Myers Squibb (BMS), AbbVie, Takeda, Novartis, Roche, and Jazz, and has received institutional research support from BMS, Novartis, and AbbVie.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal