In this issue of Blood, Abboud and colleagues1 report the results of a phase 2 trial of itacitinib given as add-on to standard graft-versus-host disease (GVHD) prophylaxis in 42 patients receiving haplo-identical stem cell transplantation (haplo-SCT) to test if cytokine release syndrome can be prevented. The authors found that itacitinib was well tolerated and was associated with low rates of GVHD and cytokine release syndrome.
Allogeneic hematopoietic SCT is for many patients with hematologic malignancies the only chance for cure. HLA-matched related donors or matched unrelated donors are preferable for SCT but not available to all patients.
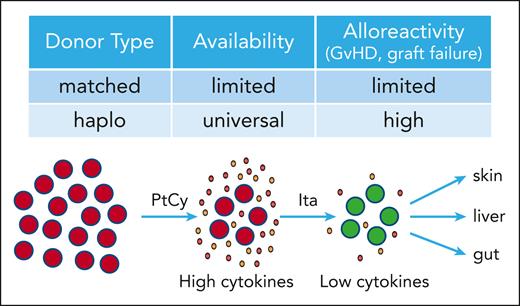
Haplo-SCT uses a half-matched transplant, usually from a family member, and thus provides rapid and near universal donor availability. However, haplo-SCT comes with increased bidirectional alloreactivity leading to cytokine release syndrome and high rates of graft failure and GVHD (see figure). Alloreactivity can be reduced in haplo-SCT by posttransplant cyclophosphamide (PtCy), which attenuates proliferating alloreactive T cells but has specific toxicities. Despite PtCy, GVHD still affects up to 50% of patients.2
Immune-cell activation and tissue damage in GVHD is driven by inflammatory cytokines that need Janus Kinases (mainly JAK1 and 2) to transmit their signals in immune cells. The JAK1/2 inhibitor ruxolitinib has demonstrated superior activity compared with investigator’s choice in patients with acute3 and chronic GVHD4 after failure of steroids, which has led to approval of ruxolitinib for the treatment of steroid-refractory acute and chronic GVHD. Itacitinib, a JAK1-specific inhibitor, in a phase 3 trial comparing itacitinib vs placebo in combination with steroids for initial treatment of acute GVHD has demonstrated an improvement in day 28 responses that did not reach statistical significance.5
Abboud et al are the first to present results of a prospective clinical trial investigating a JAK inhibitor as GVHD prophylaxis in haplo-SCT. Itacitinib was given in combination with standard GVHD prophylaxis consisting of tacrolimus, mycophenolate mofetil, and PtCy. To avoid GVHD flare, tapering of itacitinib was changed from day 100 to day 180 in the extension part of the trial to increase itacitinib exposure, which in the future might allow for earlier tapering of calcineurin inhibitors. Results were convincing: there were no graft failures, and all patients achieved full donor chimerism. None of the patients experienced grade 2 to 4 cytokine release syndrome. Cumulative incidence of grade 2 acute GVHD was 21.9% by day 100 post-SCT. The rate of grade 3 to 4 acute GVHD was 0%, which favorably compares with the expected rate of more than 15%.6 The 1-year cumulative incidence of mild and moderate to severe chronic GVHD was 7% and 5%. To put this into context, low rates of grade 3 to 4 acute GVHD (4.4%) and 1-year moderate to severe chronic GVHD (15.9%) were seen with the addition of abatacept to PtCy in haplo-SCT.7 Abatacept is a CTLA4 antibody that was recently approved by the Food and Drug Administration for prevention of acute GVHD in matched or 1-allele-mismatched unrelated donor, in the non-PtCy setting.
The results reported by Abboud and colleagues demonstrate that itacitinib can be combined with the PtCy platform without compromising the depleting effect of PtCy on alloreactive T cells (see figure). These data add a viable treatment option to prevent acute and chronic GVHD in haplo-SCT. How to proceed from here? First, the encouraging low rates of GVHD need to be confirmed in larger, randomized trials examining the role of itacitinib in the haplo-SCT setting to establish solid ground for drug approval and the evolving treatment standards. Second, lower rates of GVHD must not come at the price of higher rates of graft failure, nonrelapse mortality (NRM), and attenuated alloreactivity against the malignancy. It is encouraging that available data do not support a negative impact of treatment with JAK inhibitors on these parameters. Ruxolitinib and itacitinib do come with increased rates of toxicity, mainly thrombocytopenia and viral infections. Results of the trial by Abboud et al show clinical rates for relapse of the malignancy of 14% at 2 years and NRM of 11% at 1 year and no graft failures and thus align with previous data reported for itacitinib for initial treatment5 and ruxolitinib in steroid-refractory GVHD.3,4 However, definitive conclusions for relapse rates and NRM with itacitinib in haplo-SCT can only be made from prospective, randomized trials with sufficient follow-up. Of note, in preclinical models for ruxolitinib and baricitinib, a balanced inhibition of JAK1 and JAK2 with sparing of JAK3 has been associated with favorable GVHD activity while preserving alloreactivity against the malignancy.8,9 Itacitinib is a JAK1-specific inhibitor. We currently do not know how the lack of JAK2 activity might translate into activity or tolerability compared with ruxolitinib. Third, the activity of JAK inhibitors to prevent GVHD and cytokine release needs to be investigated in HLA-matched donor SCT. Emerging data indicate activity of JAK inhibitors in preventing GVHD.10 In this light, results of trials evaluating itacitinib (NCT04339101) and ruxolitinib (NCT06615050) after reduced-intensity conditioning SCT are eagerly awaited.
The authors have to be congratulated for this well-performed academic trial. Once confirmed, these data have the potential to change treatment standards and to ultimately reduce the risk of GVHD and cytokine release syndrome in patients receiving haplo-SCT.
Suppression of alloreactive T cells in haplo-SCT by itacitinib. Haplo-SCT provides rapid and near universal donor availability, but comes with increased alloreactivity. PtCy depletes alloreactive T cells and thereby reduces GVHD and graft failure. Itacitinib suppresses alloreactive T cells further lowering GVHD damage in target organs (skin, liver, gut).
Suppression of alloreactive T cells in haplo-SCT by itacitinib. Haplo-SCT provides rapid and near universal donor availability, but comes with increased alloreactivity. PtCy depletes alloreactive T cells and thereby reduces GVHD and graft failure. Itacitinib suppresses alloreactive T cells further lowering GVHD damage in target organs (skin, liver, gut).
Conflict-of-interest disclosure: N.v.B. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal