In this issue of Blood, Rothfuß et al report that acute myeloid leukemia (AML) blasts secrete prostaglandin E2 (PGE2), which leads to functional impairment of natural killer (NK) cells through inhibition of their Lck-dependent activation.1
NK cells are crucial players in AML immune surveillance and are the first lymphocyte subset to reconstitute after allogeneic hematopoietic stem cell transplantation, a setting in which they greatly contribute to prevent AML relapse. Moreover, efforts are ongoing to further exploit the NK-cell potential against AML through NK-cell adoptive transfer and NK cell–engaging approaches, namely chimeric antigen receptor (CAR)-NK cells, bispecific antibodies, and NK cell–targeting cytokines. All these approaches can be limited by the development of NK-cell dysfunction as a result of interaction of NK cells with AML blasts. Understanding the molecular mechanisms behind AML blasts-induced NK-cell dysfunction appears therefore essential.
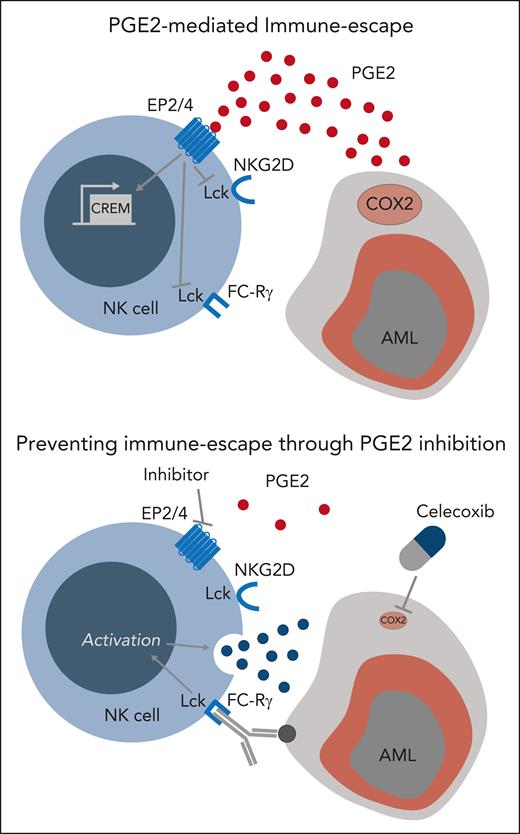
In the current study, Rothfuß et al used an unsupervised approach to identify gene sets modulated in NK cells after exposure to AML blasts and observed an upregulation of the transcription factor cyclic adenosine 5′-monophosphate (AMP)-responsive element modulator (CREM). Exposure to PGE2, a main inducer of cyclic AMP signaling in immune cells, led to increased CREM expression and recapitulated an NK-cell transcriptional signature as well as functional dysfunction similar to the ones induced by AML blasts. Moreover, the authors observed high levels of the PGE2-producing enzyme cyclooxygenase-2 (PTGES2/cyclooxygenase-2 [COX2]) gene expression as well as of PGE2 secretion by AML blasts but not by physiologic myeloid cells. Phosphoproteomic analysis showed that PGE2 inhibited Lck-dependent NK-cell activation (see figure, upper panel) by decreasing mammalian target of rapamycin and extracellular signal-regulated kinase 1/2 signaling. This work adds PGE2 to the list of AML-derived factors responsible of NK-cell dysfunction, which already includes interleukin-10 (IL-10)2 and transforming grown factor β.3 PGE2 is known to affect antitumor cytotoxic cells, namely NK cells4 and tumor-infiltrating CD8 T cells,5,6 by reducing interleukin-2 receptor common γ chain expression and IL-2 signaling. The current study provides an additional mechanism through which PGE2 affects NK-cell function, by directly interfering with its activation through Lck inhibition.
Blockade of the PGE2-EP2/4 axis prevents AML immune escape from NK-cell killing.
Blockade of the PGE2-EP2/4 axis prevents AML immune escape from NK-cell killing.
In addition, the study by Rothfuß and colleagues provides experimental evidence that the clinically employed COX2 inhibitor celecoxib has the potential to prevent AML immune escape from NK-cell killing by reducing their production of PGE2. Similar results were obtained by blocking PGE2 signaling using an experimental EP2/4 inhibitor. These results identify the PGE2-EP2/4 axis as an actionable target to prevent AML immune escape from NK-cell killing (see figure, lower panel). Unfortunately, all experiments presented in the current study were exclusively performed in vitro and no in vivo data are provided to support the feasibility of combining NK cells with PGE2-EP2/4 axis blockade in animal models of AML. However, a recent work performed by the same group in a preclinical model of metastatic melanoma supports the in vivo relevance of these results by showing that double genetic deficiency of EP2 and EP4 in NK cells prevents the development of NK-cell dysfunction and controls metastatic disease.7 Confirmation of the effect of COX2 inhibitors, such as celecoxib, in combination with NK cell–based therapies in preclinical patient-derived xenograft models of AML will be required before potentially moving this approach to the clinic.
It is now well established that NK-cell dysfunction as a result of chronic stimulation,8 inhibitory signals,3 and even PGE2 exposure7 is associated with specific epigenetic modifications responsible for a stable and only partially reversible functional alteration. It remains to be tested whether interfering with the PGE2-EP2/4 axis can reverse already established NK-cell dysfunction in addition to preventing it. This has major implications for potential clinical translation of this approach, in particular if combination with NK cell–engaging therapies targeting endogenous, and therefore already dysfunctional, NK cells is foreseen.
In summary, the work by Rothfuß et al provides solid evidence that PGE2 production by AML blasts contributes to their immune escape from NK-cell killing by inhibiting NK-cell activation. The PGE2-EP2/4 axis therefore represents a new target to prevent and potentially reverse NK-cell dysfunction in AML. These findings provide a rationale for combining pharmacological approaches targeting the PGE2-EP2/4 axis with NK cell–based therapies against AML.
Conflict-of-interest disclosure: F.S. reports institutional consulting fees from Bristol Myers Squibb/Celgene, Incyte, and Kite/Gilead; speaker fees from Kite/Gilead and Incyte; travel support from Kite/Gilead, Novartis, AstraZeneca, Neovii, and Janssen; and research funding from Kite/Gilead, Novartis, and Bristol Myers Squibb/Celgene.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal