Key Points
An unbiased, systematic screening approach revealed SMAC mimetics to be effective enhancers of NK-cell cytotoxicity in CML.
Tyrosine kinase inhibitors were found to inhibit NK-cell cytotoxicity in CML via reduction of IFN-γ expression in NK cells.
Visual Abstract
Natural killer (NK) cells have proven to be safe and effective immunotherapies, associated with favorable treatment responses in chronic myeloid leukemia (CML). Augmenting NK-cell function with oncological drugs could improve NK-cell–based immunotherapies. Here, we used a high-throughput drug screen consisting of >500 small-molecule compounds, to systematically evaluate the effects of oncological drugs on primary NK cells against CML cells. We identified second mitochondrially derived activator of caspases (SMAC) mimetics as potent enhancers of NK-cell cytotoxicity in both cell lines and primary patient samples. In contrast, several drug classes, including glucocorticoids and tyrosine kinase inhibitors such as dasatinib, inhibited NK-cell cytotoxicity. Single-cell RNA sequencing revealed drug-induced transcriptomic changes in both NK and target CML cells. SMAC mimetics upregulated NF-κB target genes in NK cells, potentially contributing to their enhanced cytotoxicity. Inhibitory drugs dexamethasone, dasatinib, and sotrastaurin prevented NK-cell transition to an activated state and suppressed the expression of interferon gamma (IFN-γ) by NK cells, thus preventing IFN-γ–mediated target cell transcriptomic response. In conclusion, we discovered that SMAC mimetics sensitize cancer cells to NK-cell–mediated killing, with potential clinical applications especially in patients with advanced phase CML.
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm characterized by the Philadelphia chromosome leading to BCR::ABL1 fusion gene.1 The development of tyrosine kinase inhibitors (TKIs) has had a significant impact on the prognosis of patients with chronic-phase (CP) CML, with the goal of treatment nowadays being TKI discontinuation and treatment-free remission.2 However, only 38% to 64% of patients attempting TKI discontinuation are able to sustain treatment-free remission.3,4 Residual leukemic stem cells (LSCs), which survive TKI treatment in vivo, may contribute to disease progression and relapse.5-7 The immune system has been proposed to play a significant role in treatment-free remission, enabling control of residual LSCs.8 Blast-phase CML (BP-CML) presents as an acute leukemia, with a median overall survival of <1 year and is often resistant to TKI therapy.9,10 BP-CML leukemia cells harbor, in addition to the BCR::ABL1 fusion, many other genetic abnormalities, which are difficult to target with available small-molecule drugs.11,12 Therefore, BP-CML represents one of the most pressing clinical challenges in CML treatment.
Natural killer (NK) cells are innate immune cells capable of killing virus-infected and malignant cells. NK-cell immunotherapies are promising novel treatments that have enabled complete remission in patients with relapsed/refractory myeloid leukemias.13 Unlike T cells, NK cells do not cause graft-versus-host disease and, thus, are a safer form of cellular immunotherapy.14,15
The significance of NK cells in patients with CML has been highlighted in several studies.16,17 A higher percentage of mature NK cells was associated with successful TKI discontinuation.18,19 Moreover, TKIs have complex interactions with NK-cell functions in CML. Dasatinib and imatinib were suggested to enhance NK-cell cytotoxicity through regulation of activating and inhibitory receptors20,21 and an increased number of NK cells was observed in patients treated with dasatinib or imatinib,22,23 but previous studies also indicated inhibitory effects on NK cells.24
In addition to cytokines and bi- and tri-specific antibodies, small-molecule drugs may enhance NK-cell cytotoxicity.25 High-throughput drug sensitivity and resistance testing (DSRT) can be used to systematically analyze the impact of several hundreds of small-molecule agents on cellular responses. Previously, the DSRT platform has been used to test the ex vivo drug sensitivity of primary leukemia and solid tumor cells.26,27 In addition, we previously showed that the DSRT platform can be applied in an immunological setting to analyze the impact of drugs on the efficacy of chimeric antigen receptor T cells.28
Here, we used the DSRT platform combined with CML and NK-cell coculture, to identify novel drugs which can impact NK-cell cytotoxicity. The effects of enhancing and inhibitory drugs were further characterized with single-cell RNA sequencing in dynamic coculture models and confirmed with primary patient samples. Our results provide insights into how NK-cell cytotoxicity can be improved by small-molecule drugs which may have implications for future clinical NK-cell immunotherapies.
Methods
More detailed descriptions of the experimental methods and analyses are available in supplemental Methods available on the Blood website.
Cell lines and NK cells
Patients
For primary patient cell cytotoxicity assays, we used viably frozen samples from CML CP (n = 5) and BP patients (n = 6). For controls, we used either fresh or frozen CD34+ cells from the bone marrows of healthy donors (n = 2). Patients gave informed consent, and the study was conducted in accordance with the Declaration of Helsinki.
NK-cell coculture drug sensitivity screens
A drug library of 527 investigational and approved oncological drugs was used. The coculture screens were performed using a luciferase-based readout of target cell viability. K562-luc cells were cocultured with NK cells and exposed to the drug library for 24 hours, after which luciferase luminescence was measured and drug sensitivity scores (DSSs) calculated.30,31 Validation screens were conducted according to the same protocol using custom drug plates containing only selected drugs. For the NK only screen, a similar protocol was used but target cells were not added, and NK-cell viability was determined using CellTiter-Glo (Promega).
Flow cytometry–based primary CML cytotoxicity assay
Frozen cells from patients with CML and healthy donors were thawed and cultured on a 96-well plate with or without NK cells in the presence of birinapant, NVP-LCL161, dexamethasone, dasatinib, imatinib, or dimethyl sulfoxide (DMSO). After 24 hours, cells were stained with antibodies for CD34, CD38, CD56, and the viability marker 7-AAD. Data were acquired using a Novocyte Quanteon flow cytometer and analyzed using FlowJo v10.9 software (BD Life Sciences) to obtain counts of viable CD34+ cells.
Colony forming assays
Healthy expanded NK cells, as in previous experiments, and CD34+ selected cells from 3 patients with CML and 2 healthy donors were thawed 3 days before the experiment and suspended in R10 and StemSpan media, respectively. NK cells and CD34+ cells were plated in a 2:1 effector-to-target (E:T) ratio with or without drug. After 24 hours from plating, cells from each well were added to MethoCult media and split into 3 wells on a 6-well plate.32 After 14 days colonies were counted according to the STEMCELL Technologies manual.
Patients’ NK cells
Mononuclear cells from fresh peripheral blood samples of patients with CML receiving imatinib treatment and healthy donors were extracted using Ficoll. After mononuclear cell separation, NK cells were extracted using the NK selection kit (Miltenyi) and left overnight in R10 media containing 10 ng/mL interleukin-2. After 24 hours, patient and healthy NK cells were plated in different E:T ratios with K562-luc cells with or without drugs. A luciferase readout was performed after 24 hours to assess target cell viability.
Single-cell RNA sequencing
K562 parental cells were cocultured with NK cells, NK cells and drugs, or with DMSO for 24 hours. After coculture, cell hashing was performed with BioLegend TotalSeqA protocol, and samples were pooled. Single-cell gene expression profiles were studied using 10x Genomics Chromium Single Cell 3′ RNA-seq platform. The sample libraries were sequenced on Illumina NovaSeq 6000 system. Data processing and analysis were performed using the 10x Genomics Cell Ranger pipeline. Cells were demultiplexed and downstream analysis was performed using the Seurat R package.33
Clustering and differential gene expression analysis
Seurat’s function FindMarkers using the Student t test followed by the Benjamini-Hochberg adjustment of P values was used to find differentially expressed genes between 2 classes (experimental conditions or clusters).
Statistical analysis
The statistical details of all experiments are reported in the text, figure legends, and figures, including statistical analyses performed, statistical significance, and sample counts.
Results
DSRT of CML cells cocultured with NK cells
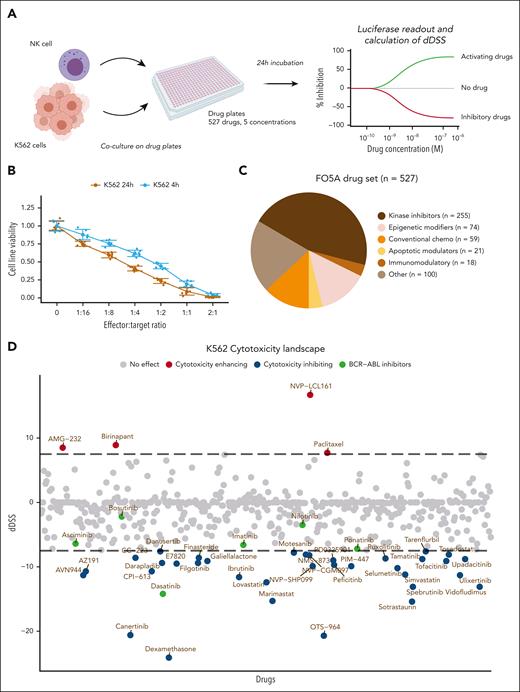
To identify drugs influencing NK-cell cytotoxicity against CML cells, we combined a high-throughput drug screen with a coculture assay using primary expanded NK cells from a healthy donor and K562 CML cells expressing luciferase (Figure 1A). K562 target cell luminescence decreased with increasing effector-target ratios, indicating accurate measurement of NK-cell–induced target cell killing (Figure 1B). We chose an E:T ratio at which 50% of target cells remained viable to observe both cytotoxicity-enhancing and -inhibiting effects. In the DSRT screen, we used a library of 527 investigational and approved molecules including kinase inhibitors, conventional chemotherapeutics, immunomodulatory agents, epigenetic regulators, and others (Figure 1C; supplemental Table 1). Target cells were cultured either with NK cells or alone for 24 hours at 5 different drug concentrations, spanning a 10 000-fold concentration range. To evaluate the drug's effect, we used a differential DSS (dDSS), a selective metric based on the area between the dose-response curves of the 2 conditions (K562+NK cells vs K562 cells alone) derived from the cell viability readout data. We also tested a 4-hour incubation time but found it insufficient to detect any effect in most drugs (Figure 1B; supplemental Figure 1; supplemental Table 2).
High-throughput drug screen identifies drugs modulating NK-cell cytotoxicity. (A) Schematic of the high-throughput coculture DSRT. (B) K562-luc cell viability with different E:T ratios of NK cells cocultured for 4 hours or 24 hours, in which bars indicate standard deviations and dots indicate the 6 technical replicates for each condition. (C) Overview of the main drug classes included in the compound library. Individual drugs are found in supplemental Table 1. (D) Overview of the landscape of drug responses in the 24-hour NK-cell cytotoxicity screen. A positive dDSS between NK-cell–treated and control drug screens indicates that the drug enhances NK-cell cytotoxicity, whereas a negative score indicates inhibition. The dotted line indicates cutoff values of 7.5 and −7.5. Drugs falling between the dotted lines are considered either only modestly effective or have no effect, whereas drugs falling outside the dotted lines are considered to have an effect.
High-throughput drug screen identifies drugs modulating NK-cell cytotoxicity. (A) Schematic of the high-throughput coculture DSRT. (B) K562-luc cell viability with different E:T ratios of NK cells cocultured for 4 hours or 24 hours, in which bars indicate standard deviations and dots indicate the 6 technical replicates for each condition. (C) Overview of the main drug classes included in the compound library. Individual drugs are found in supplemental Table 1. (D) Overview of the landscape of drug responses in the 24-hour NK-cell cytotoxicity screen. A positive dDSS between NK-cell–treated and control drug screens indicates that the drug enhances NK-cell cytotoxicity, whereas a negative score indicates inhibition. The dotted line indicates cutoff values of 7.5 and −7.5. Drugs falling between the dotted lines are considered either only modestly effective or have no effect, whereas drugs falling outside the dotted lines are considered to have an effect.
In the 24-hour screen, of the 527 screened drugs, 36 had an inhibitory effect on cytotoxicity (dDSS < −7.5), 4 drugs had an enhancing effect (dDSS > 7.5), 49 drugs had a modest inhibitory effect (−7.5 < dDSS < −5.0), 13 drugs had a modest enhancing effect (5 < dDSS < 7.5), and 426 drugs had no effect (−5 < dDSS < 5; Figure 1D; supplemental Figure 2A).
SMAC mimetics enhance NK-cell cytotoxicity, whereas dexamethasone and dasatinib show inhibitory effects
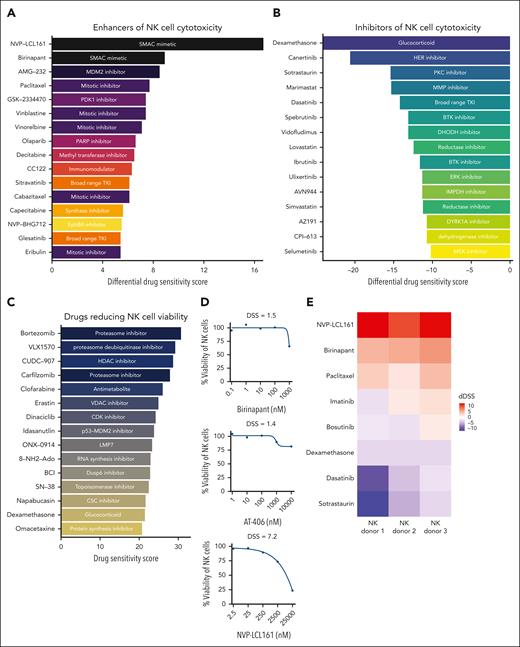
The second mitochondrially derived activator of caspases (SMAC) mimetics NVP-LCL161 and birinapant were identified as the most potent enhancers of NK-cell cytotoxicity (Figure 2A; supplemental Table 1). The third SMAC mimetic included in this drug library, AT-406, displayed NK-cell cytotoxicity–enhancing effects at the highest concentrations (supplemental Table 1; supplemental Figure 2B). SMAC mimetics are drugs which inhibit cellular inhibitor of apoptosis proteins, which are critical regulators of cell death.34 Other drugs with enhancing effects on NK-cell cytotoxicity include MDM-2 inhibitor AMG-32, vinca-alkaloid agents and taxoids (Figure 2A).
Enhancers and inhibitors of NK-cell cytotoxicity in K562 CML cells and primary CML sample. (A) Top 15 drugs most potently enhancing NK-cell cytotoxicity ordered by the dDSS). (B) Top 15 drugs most potently inhibiting NK-cell cytotoxicity, ordered by the dDSS. (C) Top 15 drugs most potently inhibiting NK-cell viability. (D) Dose-response curves showing the effects of the 3 SMAC mimetics included in the drug library on NK-cell viability. (E) Heat map showing the variability of drug responses across 3 healthy NK-cell donors from a validation drug screen. Red boxes represent enhancement of NK-cell cytotoxicity, whereas purple represents inhibition of NK-cell cytotoxicity. (F) Dose-response curves for the top drugs when validated in 3 different NK-cell donors. Orange curve and points indicate drug responses with coculture of NK cells and target cells, and the blue curve and points indicate drug responses with the target cells only. Curves are drawn using the median percent inhibition values across NK-cell donors shown as darker orange dots, with lighter orange dots indicate individual NK-cell donor percent inhibition values. BTK, Bruton tyrosine kinase; CDK, cyclin-dependant kinase; CSC, cancer stem cell; ERK, extracellular signal-regulated kinase; HDAC, histone deacetylase inhibitor; HER, human epidermal growth factor receptor; IMPDH, inosine-5'-monophosphate dehydrogenase; VDAC, voltage dependant anion channel.
Enhancers and inhibitors of NK-cell cytotoxicity in K562 CML cells and primary CML sample. (A) Top 15 drugs most potently enhancing NK-cell cytotoxicity ordered by the dDSS). (B) Top 15 drugs most potently inhibiting NK-cell cytotoxicity, ordered by the dDSS. (C) Top 15 drugs most potently inhibiting NK-cell viability. (D) Dose-response curves showing the effects of the 3 SMAC mimetics included in the drug library on NK-cell viability. (E) Heat map showing the variability of drug responses across 3 healthy NK-cell donors from a validation drug screen. Red boxes represent enhancement of NK-cell cytotoxicity, whereas purple represents inhibition of NK-cell cytotoxicity. (F) Dose-response curves for the top drugs when validated in 3 different NK-cell donors. Orange curve and points indicate drug responses with coculture of NK cells and target cells, and the blue curve and points indicate drug responses with the target cells only. Curves are drawn using the median percent inhibition values across NK-cell donors shown as darker orange dots, with lighter orange dots indicate individual NK-cell donor percent inhibition values. BTK, Bruton tyrosine kinase; CDK, cyclin-dependant kinase; CSC, cancer stem cell; ERK, extracellular signal-regulated kinase; HDAC, histone deacetylase inhibitor; HER, human epidermal growth factor receptor; IMPDH, inosine-5'-monophosphate dehydrogenase; VDAC, voltage dependant anion channel.
The immunomodulatory agent dexamethasone was the most potent inhibitor of NK-cell cytotoxicity (Figures 1D and 2B), followed by canertinib and the PKC inhibitor sotrastaurin. Dasatinib and, to an extent, ponatinib (second- and third- generation TKIs) also inhibited NK-cell cytotoxicity (Figure 1D). Other TKIs used in the CML management, imatinib and asciminib, had modest inhibitory activity (Figure 1D; supplemental Table 1), whereas bosutinib and nilotinib had negligible effects (Figure 1D; supplemental Table 1).
We performed a separate drug screen investigating the effects of the same drug panel on the viability of NK cells alone to assess whether the observed effects could be in part due to the drugs impacting NK-cell viability. Dexamethasone showed a substantial NK-cell killing effect (Figure 2C). In contrast, dasatinib and sotrastaurin had negligible effects on NK-cell viability, suggesting that they impair NK-cell cytotoxicity without killing the NK cells (supplemental Table 3). SMAC mimetics did not markedly impact NK-cell viability (Figure 2D).
Drug effects on cytotoxicity are independent of NK-cell donors
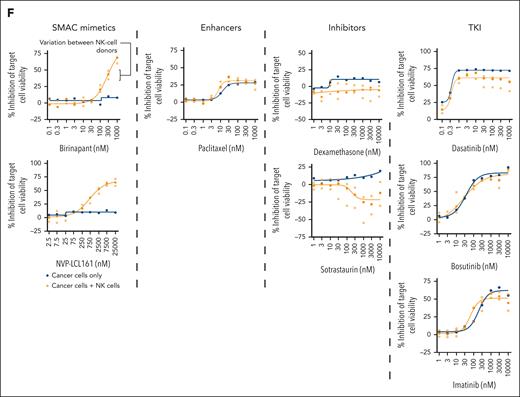
To evaluate whether the top enhancing and inhibiting drugs have consistent effects across NK cells from different donors, we performed a DSRT experiment with 3 different NK donors using a custom panel of drugs including birinapant, NVP-LCL161, dexamethasone, sotrastaurin, paclitaxel, dasatinib, and imatinib across 9 concentrations. Despite some discrepancies, results were in line with the initial screening results and drug responses were similar between different donors, indicating that drug-induced effects on cytotoxicity were not specific to NK-cell donors (Figure 2E-F).
SMAC mimetics sensitize primary cells from patients with CML to NK cells
We next assessed whether the identified drugs have consistent effects on the interaction of NK cells with primary patient-derived CML cells. We cultured primary CML CD34+ cells with NK cells for 24 hours in varying E:T ratios with the drugs birinapant, NVP-LCL161, dasatinib, imatinib, and dexamethasone at clinically relevant concentrations. After 24 hours, we measured the viability of CD34+ cells using flow cytometry. NK cells efficiently killed primary CD34+ CML cells with increasing E:T ratios (Figure 3A). Coculture of NK and CML cells at most of the tested E:T ratios in the presence of birinapant resulted in a significantly lower viability of CML cells than CML cells exposed only to NK cells in the same E:T ratios (P < .05; Figure 3B). When BP and CP samples were analyzed separately, the effects were more significant in BP samples than in CP samples (supplemental Figure 3A). Similar NK-cell enhancing effects were seen with SMAC mimetic NVP-LCL161, although the results were not statistically significant (supplemental Figure 3B). Birinapant alone appeared to have a modest direct cytotoxic effect on the viability of CML cells at 24 hours; however, this varied between patients (Figure 3C).
Effects of birinapant on NK cells in primary samples from patients with CML. (A) Lineplot of NK-cell cytotoxicity at different E:T ratios in 5 CP and 6 BP patient samples. E:T ratio of 0 represents a condition containing no NK cells, only target CD34+ CML cells. CD34+ counts have been normalized to this no-NK control. (B) Box plot showing drug sensitivity profiling of primary CML CD34+ cells’ sensitivity to NK cells at different E:T ratios with and without drug treatment ex vivo. Dots represent normalized CML CD34+ cells of all CP and BP patients after 24-hour coculture with NK cells. Median, interquartile ranges, minima, and maxima are shown. Cells are normalized by dividing average of the 3 technical replicates for each condition from each patient by the corresponding average of CML cells–only condition for each patient. Control CD34+ patient cells are represented by the dashed line. P values have been calculated with a paired t test. (C) Lineplot of CD34+ cell viability of 2 patients at different E:T ratios, 1 (left) being highly sensitive to birinapant treatment alone, and the other (right) being resistant to birinapant treatment alone. 0 represents control, no NK cells. (D) Box plot showing drug sensitivity profiling of 2 healthy donor bone marrow CD34+ cells’ sensitivity to NK cells at different E:T ratios with and without drug treatment ex vivo. Dots represent normalized healthy CD34+ cells of the 2 donors after 24-hour coculture with NK cells. Median, interquartile ranges, minima, and maxima are shown. Cells are normalized by dividing the average of the 3 technical replicates for each condition from each donor by the corresponding average of CD34+ cells–only condition for each patient. Control CD34+ cells are represented by the dashed line. (E) Bar plot of normalized total colony counts from a colony forming assay done on 2 healthy samples and 3 CML samples. Total colony counts from each condition have been normalized to the CD34-DMSO condition within each sample. (F) Box plot showing effect of drug treatment on the efficacy of unexpanded NK cells at an E:T ratio of 2:1 from 3 patients with CML, receiving imatinib (right panel), healthy control NK cells from 3 healthy donors (middle panel), and control K562-luc cells exposed only to drug and not NK cells. Median, interquartile ranges, minima, and maxima are shown.
Effects of birinapant on NK cells in primary samples from patients with CML. (A) Lineplot of NK-cell cytotoxicity at different E:T ratios in 5 CP and 6 BP patient samples. E:T ratio of 0 represents a condition containing no NK cells, only target CD34+ CML cells. CD34+ counts have been normalized to this no-NK control. (B) Box plot showing drug sensitivity profiling of primary CML CD34+ cells’ sensitivity to NK cells at different E:T ratios with and without drug treatment ex vivo. Dots represent normalized CML CD34+ cells of all CP and BP patients after 24-hour coculture with NK cells. Median, interquartile ranges, minima, and maxima are shown. Cells are normalized by dividing average of the 3 technical replicates for each condition from each patient by the corresponding average of CML cells–only condition for each patient. Control CD34+ patient cells are represented by the dashed line. P values have been calculated with a paired t test. (C) Lineplot of CD34+ cell viability of 2 patients at different E:T ratios, 1 (left) being highly sensitive to birinapant treatment alone, and the other (right) being resistant to birinapant treatment alone. 0 represents control, no NK cells. (D) Box plot showing drug sensitivity profiling of 2 healthy donor bone marrow CD34+ cells’ sensitivity to NK cells at different E:T ratios with and without drug treatment ex vivo. Dots represent normalized healthy CD34+ cells of the 2 donors after 24-hour coculture with NK cells. Median, interquartile ranges, minima, and maxima are shown. Cells are normalized by dividing the average of the 3 technical replicates for each condition from each donor by the corresponding average of CD34+ cells–only condition for each patient. Control CD34+ cells are represented by the dashed line. (E) Bar plot of normalized total colony counts from a colony forming assay done on 2 healthy samples and 3 CML samples. Total colony counts from each condition have been normalized to the CD34-DMSO condition within each sample. (F) Box plot showing effect of drug treatment on the efficacy of unexpanded NK cells at an E:T ratio of 2:1 from 3 patients with CML, receiving imatinib (right panel), healthy control NK cells from 3 healthy donors (middle panel), and control K562-luc cells exposed only to drug and not NK cells. Median, interquartile ranges, minima, and maxima are shown.
Dexamethasone and dasatinib inhibited NK-cell cytotoxicity against primary CD34+ CML cells, with dasatinib having the strongest cytotoxicity-inhibiting effect (supplemental Figure 4Bi-iii; supplemental Figure 5A-C; supplemental Figure 6A-C). Imatinib had both modestly enhancing and inhibiting effects depending on the E:T ratios used, possibly due to the direct effect of imatinib on target cells (supplemental Figure 4Bi-iii; supplemental Figure 5A-C; supplemental Figure 6A-C). Taken together, our data suggest that findings from the high-throughput screen are generalizable to patient-derived cells.
SMAC mimetics in combination with NK treatment reduce colony forming potential of CML cells
We next wanted to assess whether SMAC mimetic treatment influences the colony forming capacity of both healthy and CML CD34+ cells. We cultured NK cells and CD34+ cells with or without birinapant at an E:T ratio of 2:1 for 24 hours, after which cells were suspended into MethoCult, and colonies were counted at day 14. The effect of birinapant alone was also tested. In the healthy samples, there were no notable differences in the total number of colonies between the DMSO and birinapant conditions (Figure 3E). Furthermore, in the presence of NK cells, birinapant treatment did not have a significant impact on the total colony counts (Figure 3E).
As with healthy CD34+ cells, birinapant treatment alone did not impact the total colony counts in the CML samples (Figure 3E). However, in the presence of NK cells, birinapant treatment reduced up to 57% the number of colonies in the CML samples (median, 53.9% [range, 29.4%-56.9%]; Figure 3E). Based on this we conclude that birinapant treatment in combination with NK-cell therapy has inhibitory effects on the stem cell compartment in CML.
SMAC mimetic treatment can also enhance the cytotoxic effects of NK cells derived from patients with CML
To test whether SMAC mimetic treatment can enhance the function of NK cells derived from patients with CML, we extracted primary, nonexpanded NK cells from patients with CP CML receiving imatinib treatment and repeated the coculture experiments with K562-luc cells. We found that both birinapant and NVP-LCL161 were able to effectively enhance the cytotoxic function of patient-derived NK cells at all tested E:T ratios (Figure 3F; supplemental Figure 7A-C). Similarly, birinapant and NVP-LCL161 also boosted the killing efficacy of nonexpanded NK cells from healthy donors (Figure 3F; supplemental Figure 7A-C)
Single-cell RNA sequencing reveals drug-induced NK-cell states
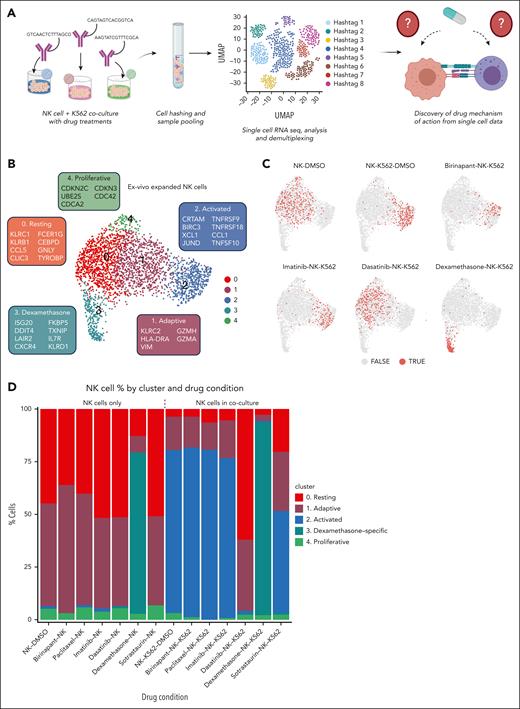
Next, we performed coculture assays with a single-cell RNA sequencing readout to comprehensively map the effects of the identified drugs on both NK and cancer cell states and to identify potential mechanisms-of-action. We aimed to define transcriptional changes caused by K562-NK interaction and how this was impacted by drug treatment. We selected top enhancing and inhibitory drugs from the previous drug screen and performed cocultures in different conditions: drugs with target cells only, drugs with NK cells only, and drugs with coculture, including DMSO as a control for all conditions. Antibody oligonucleotide tags were used to multiplex cells from different conditions and perform a pooled single-cell experiment. After single-cell sequencing, cells were demultiplexed and assigned to their drug conditions for downstream analysis (Figure 4A).
Phenotypes of NK cells identified by single-cell RNA sequencing with and without drug treatment. (A) Schematic of the single-cell sequencing experiment. (B) Uniform manifold approximation and projection (UMAP) of primary expanded NK cells from all conditions. The genes in boxes indicate marker genes for each cluster. Genes are selected from a list of differentially expressed genes based on significant P value, fold change, and biological relevance. (C) UMAPs of expanded NK cells from selected drug conditions. Dots colored light red (TRUE) represent NK cells from the respective condition, and gray dots (FALSE) represent NK cells from all other conditions. (D) Bar plot showing percentages of NK cells in each cluster by experimental/drug condition.
Phenotypes of NK cells identified by single-cell RNA sequencing with and without drug treatment. (A) Schematic of the single-cell sequencing experiment. (B) Uniform manifold approximation and projection (UMAP) of primary expanded NK cells from all conditions. The genes in boxes indicate marker genes for each cluster. Genes are selected from a list of differentially expressed genes based on significant P value, fold change, and biological relevance. (C) UMAPs of expanded NK cells from selected drug conditions. Dots colored light red (TRUE) represent NK cells from the respective condition, and gray dots (FALSE) represent NK cells from all other conditions. (D) Bar plot showing percentages of NK cells in each cluster by experimental/drug condition.
We used unsupervised clustering to define the NK-cell states induced by coculture and drug treatments. Five distinct NK-cell states were identified: resting (cluster 0) and adaptive (cluster 1) states, enriched in untreated NK cells; activated (cluster 2) state induced by coculture; dexamethasone-specific (cluster 3) state; and proliferative state (cluster 4; Figure 4B). Cluster 0 expressed markers CLIC3, FCER1G, CEBPD, TC2N, CCL5, and markers of resting ex vivo–expanded NK cells including KLRB1 and KLRC1,35 whereas cluster 1 expressed the adaptive NK-cell markers KLRC2, HLA-DRA, and GZMH35,36 (Figure 4B; supplemental Figure 9C). Several markers of clusters 0 and 1 were shared, including GZMA, NKG7, CCL5, indicating similar phenotypic profiles or a continuum of resting to adaptive state (supplemental Figure 9C). Activated cluster 2 expressed CRTAM, CCL1, TNFRSF9, and TNFRSF18 as previously observed35,37 (Figure 4B). NK cells in cluster 3 expressed TXNIP, IL7R, and CXCR4, whereas cluster 4 expressed cell cycle–related genes such as CDKN2C, CDKN3, and CDC42 (Figure 4B).
Dasatinib and dexamethasone impair NK-cell activation
Control NK cells cultured alone in DMSO were mostly in the resting cluster (cluster 0, 44%) and adaptive (cluster 1, 48%) states (Figure 4C-D). When cocultured with K562 target cells, NK cells showed a strong activation response and >70% of NK cells occupied the activated state (cluster 2; Figure 4C-D). Drugs alone with NK cells, in the absence of target K562 cells, did not appear to alter the state of NK cells, except for dexamethasone, which induced a dexamethasone-specific state (Figure 4C-D). The dexamethasone-induced state expressed high levels of the glucocorticoid chaperone FKBP5 and IL7R, the latter previously found to promote survival of CD56bright NK cells38 (Figure 4B; supplemental Figure 10B).
Interestingly, dexamethasone also drove NK cells in coculture into a distinct dexamethasone-specific state, instead of the activation state. However, within the dexamethasone cluster, a modest level of activation response was observed in the subcluster of NK cells exposed to dexamethasone and NK cells (supplemental Figures 10A and 11A-B). The levels of expression of activation markers in a small number of dexamethasone-exposed cells was higher than control NK cells cultured alone in DMSO but lower than cocultured NK cells (supplemental Figure 11A). In comparison, NK cells exposed to dasatinib did not form subclusters and dasatinib exhibited a higher level of inhibition of activation markers than dexamethasone (supplemental Figure 10A-B). In conclusion, the impaired NK-cell activation provides a plausible mechanism-of-action for the cytotoxicity-inhibiting drugs.
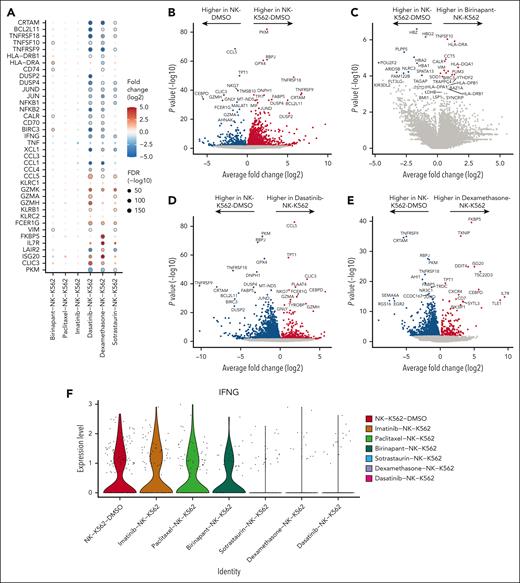
SMAC mimetics induce TRAIL expression and NF-κB signaling in tumor-exposed NK cells
Next, we performed a differential gene expression analysis for each experimental condition to identify drug-induced effects beyond broad cell state transitions (Figure 5A). In the K562 + NK-cell coculture, the most upregulated genes in NK cells were TNFRSF18, TNFRSF9, BCL2L11, and CRTAM, which are all markers of activation in NK cells (Figures 4B and 5B; supplemental Figures 9C and 10B). Although these activation markers were unaltered by birinapant, the death receptor ligand TNFSF10 and major histocompatibility complex (MHC) class II molecule HLA-DRA were induced by birinapant in cocultured NK cells (Figures 5A,C). The upregulation of TNFSF10 (TRAIL) may contribute to the increased cytotoxicity by increasing death receptor–mediated apoptosis in the target cells.39,40 The NF-κB target genes BIRC3 and the HLA II-related CD7441 were also among birinapant-induced genes, implying NF-κB activation by birinapant42 (Figures 5A,C).
Transcriptional changes identified in expanded NK cells caused by drug and target cell coculture. (A) Dot plot of selected genes upregulated and downregulated in NK cells exposed to K562 and drugs. Transcriptional data used for comparison are NK cells from coculture alone (NK-K562-DMSO). Dots encircled by gray indicate false discovery rate (FDR) <0.1. Magnitudes of gene fold changes are depicted by dot color, and the magnitude of FDR is depicted by dot size. FDR was calculated using the Benjamini-Hochberg (BH) test. (B-E) Volcano plots of differentially expressed genes from selected conditions of NK-target cell coculture and drug. Genes with an adjusted P value (BH) < .1 are colored red or blue. (F) Violin plot of IFNG expression in the NK coculture conditions.
Transcriptional changes identified in expanded NK cells caused by drug and target cell coculture. (A) Dot plot of selected genes upregulated and downregulated in NK cells exposed to K562 and drugs. Transcriptional data used for comparison are NK cells from coculture alone (NK-K562-DMSO). Dots encircled by gray indicate false discovery rate (FDR) <0.1. Magnitudes of gene fold changes are depicted by dot color, and the magnitude of FDR is depicted by dot size. FDR was calculated using the Benjamini-Hochberg (BH) test. (B-E) Volcano plots of differentially expressed genes from selected conditions of NK-target cell coculture and drug. Genes with an adjusted P value (BH) < .1 are colored red or blue. (F) Violin plot of IFNG expression in the NK coculture conditions.
Cytotoxicity-inhibiting drugs impair IFNG induction in NK cells
Dasatinib, dexamethasone, and sotrastaurin induced largely similar transcriptomic alterations, consistent with their inhibitory effect on NK-cell cytotoxicity. These included downregulation of activation markers CRTAM, TNFRSF9, TNFRSF18, BCL2L11, and BIRC3 in cocultured NK cells (Figures 5A,D-E). In addition, dasatinib, dexamethasone, and sotrastaurin all downregulated the expression of IFNG, implying reduced interferon gamma (IFN-γ) production (Figure 5F). The glucocorticoid chaperone FKBP543 was the most upregulated gene in dexamethasone-exposed cocultured cells, reflecting a dexamethasone-specific response (Figure 5E). FKBP5 has been previously implicated in several inflammation and cancer-related signaling pathways.44 The effect of imatinib on differential gene expression of cocultured NK cells was minimal, reflecting the limited impact on cytotoxicity (supplemental Figure 14B). Taken together, we infer that dexamethasone, dasatinib and, to an extent, sotrastaurin exert their cytotoxicity-inhibiting effects through reduction of NK-cell activation.
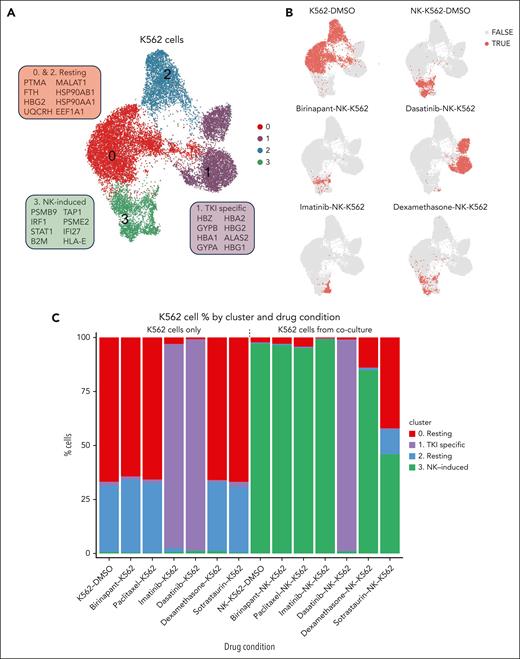
NK-induced phenotype in K562 cells identified by unsupervised clustering
We then investigated the phenotypes induced in target K562 cells by coculture with NK cells and different drugs. Unsupervised clustering revealed 4 clusters of K562 cells (Figure 6A). Cluster 1 was a TKI-specific cluster, which was enriched in cells from dasatinib-K562, dasatinib-K562-NK and imatinib-K562 conditions, but only few cells from imatinib-K562-NK condition. Clusters 0 and 2 displayed expression profiles depicting resting state K562 cells. InferCNV45 analysis indicated that the difference between the 2 clusters could be explained by a genetic subclone (supplemental Figure 17A), but we did not find evidence that this would have significant effects on downstream analysis, as the gene expression changes induced by the different conditions were consistent (supplemental Figure 17B). Most cells (>90%) in all K562-drug conditions, besides TKI treated cells, without NK exposure belonged to resting clusters 0 and 2 collectively. K562 cells from DMSO-K562-NK, birinapant-K562-NK, paclitaxel-K562-NK, dexamethasone-K562-NK, and imatinib-K562-NK belonged mostly to the NK-induced cluster (cluster 3). Although dexamethasone and dasatinib were both found to be cytotoxicity inhibitors, under dexamethasone exposure, NK cells and K562 were able to interact and K562 cells entered the NK-induced cluster, even though the NK cells did not functionally appear to be activated. However, K562 cells exposed to dasatinib and NK cells did not show NK-induced response, and remained in the state they were prior to NK-cell exposure (TKI cluster). K562 cells exposed to sotrastaurin and NK cells were mainly in the NK-induced cluster (cluster 3, 45.8%) and the resting cluster (cluster 0, 42%; Figure 6C). Our findings indicate subtle differences in the mechanisms for cytotoxicity inhibition, with dexamethasone potentially allowing limited NK-K562 interaction but blocking K562 killing also by other means such as impairing NK-cell viability, whereas dasatinib appears to entirely prevent NK cell–K562 interaction.
Phenotypes of K562 CML cells identified by single-cell RNA sequencing with and without drug treatment. (A) UMAP of K562 cells from all conditions. The genes in boxes indicate marker genes for each cluster. Genes are selected from a list of differentially expressed genes based on significant P value, fold change, and biological relevance. (B) UMAPs of K562 cells from selected drug conditions. Dots colored red (TRUE) represent K562 cells from the respective condition, and gray dots (FALSE) represent K562 cells from all other conditions. (C) Bar plot showing percentages of K562 cells in each cluster by experimental/drug condition.
Phenotypes of K562 CML cells identified by single-cell RNA sequencing with and without drug treatment. (A) UMAP of K562 cells from all conditions. The genes in boxes indicate marker genes for each cluster. Genes are selected from a list of differentially expressed genes based on significant P value, fold change, and biological relevance. (B) UMAPs of K562 cells from selected drug conditions. Dots colored red (TRUE) represent K562 cells from the respective condition, and gray dots (FALSE) represent K562 cells from all other conditions. (C) Bar plot showing percentages of K562 cells in each cluster by experimental/drug condition.
Activating drugs promote IFN-γ response and SMAC mimetics induce NF-κB target genes in K562 cells
We then examined the differentially expressed genes from each drug condition. Genes induced in K562 cells upon exposure to only NK cells included immunoproteasome-associated genes (PSME1, PSME2, PSMB1), IFN-γ pathway genes (IFI27, IFITM3, ISG15, STAT1), MHC-I genes (HLA-E, HLA-B, HLA-C, B2M), and the death receptor ligand TNFSF10 (Figure 7A-B; supplemental Figure 18B). A pathway analysis validated that the IFN-γ response pathway was the most upregulated pathway in K562 cocultured without drugs (supplemental Figure 19G).
Transcriptional changes identified in K562 target cells caused by drug and NK-cell coculture. (A) Dot plot of selected genes upregulated and downregulated in K562 cells exposed to NK cells and drugs. Transcriptional data used for comparison are K562 cells from coculture alone (NK-K562-DMSO). Dots encircled by gray indicate FDR <0.1. Magnitudes of gene fold changes are depicted by dot color, and the magnitude of FDR is depicted by dot size. FDR was calculated using the BH test. (B-C) Volcano plots of differentially expressed genes from selected conditions of NK-target cell coculture and drug. Genes with an adjusted P value (BH) < .1 are colored red. (D) Top upregulated and downregulated HALLMARK pathways in K562 cells in birinapant-K562-NK condition as compared with K562 cells from K562-NK-DMSO control condition (E-F) Volcano plots of differentially expressed genes from selected conditions of NK-target cell coculture and drug. Genes with an adjusted P value (BH) < .1 are colored red or blue. (G) Top upregulated and downregulated HALLMARK pathways in K562 cells in dasatinib-K562-NK condition (left) and imatinib-K562-NK condition (right).
Transcriptional changes identified in K562 target cells caused by drug and NK-cell coculture. (A) Dot plot of selected genes upregulated and downregulated in K562 cells exposed to NK cells and drugs. Transcriptional data used for comparison are K562 cells from coculture alone (NK-K562-DMSO). Dots encircled by gray indicate FDR <0.1. Magnitudes of gene fold changes are depicted by dot color, and the magnitude of FDR is depicted by dot size. FDR was calculated using the BH test. (B-C) Volcano plots of differentially expressed genes from selected conditions of NK-target cell coculture and drug. Genes with an adjusted P value (BH) < .1 are colored red. (D) Top upregulated and downregulated HALLMARK pathways in K562 cells in birinapant-K562-NK condition as compared with K562 cells from K562-NK-DMSO control condition (E-F) Volcano plots of differentially expressed genes from selected conditions of NK-target cell coculture and drug. Genes with an adjusted P value (BH) < .1 are colored red or blue. (G) Top upregulated and downregulated HALLMARK pathways in K562 cells in dasatinib-K562-NK condition (left) and imatinib-K562-NK condition (right).
Addition of birinapant to K562–NK-cell coculture resulted in increased expression of NF-κB target genes BIRC3, TNFSF10, and CD74 in K562 cells (Figure 7A,C). Although individual IFN-γ genes were not among the top expressed genes, many genes in this pathway were differentially expressed between birinapant-K562-NK and K562-NK conditions, and a pathway analysis showed the IFNG pathway to be upregulated by birinapant (Figure 7D). The TNFA-NFKB signaling pathway was also upregulated by birinapant (Figure 7D) and NFKB2, NFKBIE and NFKBIA were among the top significant differentially upregulated genes by birinapant in coculture (Figure 7C). Birinapant alone, in the absence of NK cells, was also able to upregulate NF-κB pathway genes in target K562 cells (supplemental Figure 20A). Upregulation of the IFNG pathway and the sensitization via the tumor necrosis factor (TNF) pathway are both potential mechanisms underlying the cytotoxicity-enhancing effects of SMAC mimetics.
Impact of NK-cell cytotoxicity–inhibiting drugs on target K562 cells
Finally, we investigated differentially expressed genes in NK-K562 cocultures exposed to cytotoxicity-inhibiting drugs. Dexamethasone, sotrastaurin, and dasatinib significantly downregulated genes of the IFNG pathway (IRF1), MHC class I molecules (HLA-E), proteasome-related genes (PSME1,2), as well as STAT1 (Figure 7A,E-F; supplemental Figure 22B-D), when compared to the control DMSO-K562-NK–cell condition. Imatinib did not show similar effects, consistent with its limited impact on NK-cell cytotoxicity. According to a pathway analysis, IFN-γ response was downregulated by dexamethasone, sotrastaurin, and dasatinib, but upregulated by imatinib (supplemental Figure 19C-F). The impaired IFN-γ responses observed in target cells upon inhibitory drug treatment are consistent with the reduced IFNG expression in NK cells in the respective drug conditions (Figure 5F).
Discussion
We aimed to discover drug classes affecting the cytotoxicity of NK cells against CML cells and thus lay a foundation for future immunotherapy applications. We found both cytotoxicity activating and inhibiting drug classes. A key finding was that SMAC mimetics were able to enhance NK cytotoxicity across cell lines, patient samples, and NK-cell donors.
We identified the SMAC mimetics birinapant and LCL-161 as potent enhancers of NK-cell cytotoxicity against CML cells. SMAC mimetics were previously shown to be effective as part of combination immunotherapies in multiple myeloma and various solid tumors.46,47 Additionally, previous studies by our group and others have also found SMAC mimetics to be effective enhancers of T-cell and chimeric antigen receptor T-cell cytotoxicity.28,48-50 Reassuringly, a clinical phase 1 study reported a promising safety profile for birinapant.51 Hematologic toxicities were mostly mild to moderate, and all patients who experienced a hematologic adverse event were able to continue treatment either at the same or lower dose. In concordance, our results show that although birinapant exerts some direct toxic effects on healthy CD34+ cells, it does not significantly alter their colony-forming ability. Thus, although birinapant’s therapeutic benefit as a single agent was limited, it may be utilized in combination regimens.51
Single-cell RNA sequencing data suggests that SMAC mimetics may induce an immune-inflamed phenotype in NK cells in addition to sensitizing CML cells to NK-cell cytotoxicity. While coculture alone was able to induce MHC II expression in K562 cells, the expression of the MHC II molecule CD74 was significantly higher in cells exposed to birinapant. When applied in vivo, upregulation of MHC molecules could also potentially increase recognition of tumor cells by T cells and contribute to tumor elimination. A previous study found that CML leukemic stem cells may use downregulation of MHC class II molecules, such as CD74, as a mechanism of immune evasion.52 Drugs, such as birinapant, might therefore be used to improve CML LSC immunogenicity by upregulation of MHC class II. However, these effects were limited to coculture conditions alone, indicating that the effect of birinapant on MHC class II expression requires NK-cell–target-cell interaction. It has been suggested that TRAIL, upregulated by birinapant treatment, may induce NF-κB dependent upregulation of cytokines, which may increase the chemotaxis of NK and T cells.53
A study by Pan et al highlights the intriguing possibility of serial killing by NK cells.54 Future studies could explore whether SMAC mimetic-priming enhances later NK-cell killing or improves NK-cell persistence in vivo, which is clinically relevant given that multiple doses of NK cells are often used in allogeneic therapy due to their poor in vivo persistence.
In our patient validations, we observed significantly increased NK-cell killing at lower E:T ratios, particularly 2:1. This suggests that SMAC mimetics may enhance NK killing more effectively at these lower ratios, potentially through synergistic effects via the mitochondrial apoptosis pathway, aligning with previous findings.54 These lower ratios may be more physiologically relevant than the higher E:T ratios typically used in NK-cell research, such as 4:1 or 10:1. In vivo, NK cells constitute about 5% to 20% of circulating lymphocytes, totaling roughly 2 billion in a healthy human.55 Patients with CML at diagnosis can have white blood counts ranging from 5 × 104/μL in asymptomatic patients to 1 × 106/μL in symptomatic patients, of which up to 5% will be blasts in the patients with CP, translating to an estimated 25 to 500 billion leukemic cells at diagnosis. Thus, in vivo, without exogenous NK treatment, the E:T ratios are quite small, and NK cells are outnumbered by CML cells. As for adoptive NK-cell therapy, doses of NK cells ranging from 1 × 106 cells per kg to 1 × 108 cells per kg have been reported, with doses most commonly in the 1 × 107 range.13,56-58 These NK-cell doses would result in E:T ratios from as small as 1:71 to even 2:1, with median values being around 1:2. However, in many in vitro NK studies, even higher E:T ratios such as 3:1 or 10:1 have been used.57 Thus, combining SMAC mimetics with NK cells may enable responses at physiologically relevant and clinically achievable NK-cell doses.
Inhibitory drugs downregulated IFNG in NK cells and thus IFN-γ response in target cells. In addition to its immunomodulatory effects,59,60 IFN-γ is able to directly induce apoptosis in target cancer cells.61-64 Thus, modulation of IFNG expression may be an important mechanism of drug-induced cytotoxicity enhancement or inhibition. We infer from the single-cell analysis that both upregulation of the IFN-γ pathway as well as sensitization via the TNF pathway are potential mechanisms associated with the cytotoxicity-enhancing effects of SMAC mimetics.
Dasatinib, used in the first and second line treatment of CML, was found to be strongly inhibit NK-cell cytotoxicity, in line with previous in vitro studies.24 Both dasatinib and imatinib were also shown to inhibit the expansion of T cells and impair their function.65-68 This is somewhat in contrast to what has been observed in patients. Clinical data revealed that TKIs, particularly dasatinib, can cause the expansion of mature CD8+ cytotoxic T-cell populations and NK cells, which is associated with favorable response.20,22,23 The discrepancies between in vitro and in vivo results may be partly explained by short half-life of the drug and limited duration of the cytotoxicity-inhibiting effects.69 In addition, dasatinib induces strong mobilization of cytotoxic T and NK cells in the circulation, which may affect their functionality.70
Our results also highlight the significance of the immune-inhibitory effects of glucocorticoids. Dexamethasone, a widely used drug in the chemotherapeutic regimes of many blood cancers, was the most potent inhibitor of NK-cell cytotoxicity in CML, in addition to having direct NK-cell killing effects. Further studies developing glucocorticoid-receptor knockout NK cells could be considered to avoid glucocorticoid-induced NK-cell suppression. We also identified the PKC inhibitor sotrastaurin as an inhibitor of NK cytotoxicity. This is in accordance with previous studies showing that the PKC pathway is important for NK antitumor activity by mediating IFN-α signaling.71,72 Interestingly, dexamethasone and dasatinib appeared to inhibit NK-cell cytotoxicity at different stages, with dexamethasone potentially allowing limited NK-K562 interaction but blocking NK-cell cytotoxicity, whereas dasatinib appears to completely prevent NK cell–K562 interaction.
We validated our findings also in primary samples from patients with CML, including a sample from a patient with BP-CML with a TKI resistance mutation. We found that NK cells can effectively kill CML cells, and SMAC mimetic treatment enhanced this killing. The observed effects were more pronounced in BP patient samples. Thus, our findings provide a rationale for patients with BP-CML, including patients with TKI mutations, as potential candidates for adoptive NK-cell therapies.
The high-throughput approach we used was systematic and reduced selection bias compared to previous studies where individual selected drugs were tested, setting an example for future efforts in understanding drug effects on NK-cell functions. Our study successfully detected NK-cell cytotoxicity–modulating drugs and the use of single-cell sequencing enabled discovery of drug-induced changes in both NK and cancer cell types.
In conclusion, our results lay a foundation for the use of NK-cell–cancer drug combination therapies in the treatment of CML. However, further studies are warranted to assess the impact of birinapant in preclinical in vivo models also taking into account the heterogeneous tumor microenvironment and possible off-target effects on other immune cells. Furthermore, the clinical feasibility of the combination approach requires further studies, including identifying the patient groups for which the use of SMAC mimetics would be the most effective, as well as the best treatment stage and conditions, including whether the drugs should be administered in combination with NK-cell infusions or to enhance patients’ own immune cells. In addition, future directions include investigating whether the identified drugs can also enhance NK-cell cytotoxicity in other malignancies beyond CML.
Acknowledgments
The authors thank Laura Turunen, Jani Saarela, Katja Suomi, and Maria Nurmi of the High-Throughput Biomedicine Unit and the personnel of the Sequencing Laboratory at the Institute of Molecular Medicine Finland. Primary chronic myeloid leukemia samples were provided by the Finnish Hematology Registry and Clinical Biobank (FHRB). The FHRB biobank is supported by the Finnish Association of Hematology, Finnish Red Cross Blood Service, Institute for Molecular Medicine Finland, and participating hospitals in Finland. The authors thank all the patients for their generous participation. The authors acknowledge the Biomedicum Helsinki Flow Cytometry Core Unit supported by Biocenter Finland and the Helsinki Institute of Life Science (HiLIFE), and the Institute for Molecular Medicine Finland Single-Cell Analytics and High-throughput Biology and Sequencing Units. The authors acknowledge the computing resources provided by the IT Center for Science Ltd.
This study was supported by grants from Cancer Foundation Finland, the Jane and Aatos Erkko Foundation, the Research Council of Finland, the Sigrid Juselius Foundation, the Leukemia and Lymphoma Society (grant number 6683-24), the Gyllenberg Foundation, state funding for the University-level Health Research in Finland, an investigator initiated grant from Pfizer, the Nordic Cancer Union, the National Cancer Institute, National Institutes of Health (R01 CA276156), and HiLIFE fellow funds. The iCAN project is funded by the Research Council of Finland (grant number 320185), the University of Helsinki, Helsinki University Hospital, and the industry partner Boehringer Ingelheim. P.N. was supported by grants from the Biomedicum Helsinki Foundation, the Emil Aaltonen Foundation, the Finnish Medical Society, the Orion Research Foundation, the Finnish Hematology Association, and the Blood Diseases Research Foundation.
Authorship
Contribution: P.N., O.D., and S.M. conceived and designed the study; P.N. performed the drug profiling experiments, patient validation experiments, and analyzed the data; J.B. performed the single-cell RNA sequencing and validation experiments; E.J. and E.L. analyzed single-cell RNA sequencing data; A.I. and D.D. participated in drug profiling data analysis; J.K. generated the K562-luciferase cell line and performed cell line authentication as well as participated in single-cell RNA sequencing experiments and testing of patients’ own NK cells; K.S. performed NK-cell expansion; S.A.-A. provided primary patient samples and participated in patient validation experiment design, colony forming assay experiment design and data analysis; S.F. participated in performing patient validations and data analysis; H.H.-H. provided primary patient samples; M.M. provided samples from healthy donors; H.K. participated in colony forming assays; T.K. and H.L. participated in drug profiling experiments, NK-cell expansions, and colony forming assays; D.A.L. provided expanded NK cells and assistance with NK-cell expansion methods, T.A. designed and supervised drug profiling data analyses; O.D. and S.M. designed and supervised the study; P.N wrote the manuscript together with O.D. and S.M; and all authors contributed to writing the paper and approved the final version.
Conflict-of-interest disclosure: S.M. has received honoraria and research funding from Novartis, Pfizer, and Bristol Myers Squibb; and honoraria from Dren Bio (not related to this study). H.H.-H., on behalf of the Nordic CML study group, has received research funding from Bristol Myers Squibb, Novartis, Incyte, Pfizer, and Austrian Orphan Pharma, unrelated to this study; and has received lecture fees from Incyte. O.D. has received research funding from Gilead Sciences and Incyte, unrelated to this work; and personal fees from Sanofi, unrelated to this work. The remaining authors declare no competing financial interests.
A complete list of the members of the iCAN Study Group appears in the supplemental Appendix.
Correspondence: Satu Mustjoki, Hematology Research Unit Helsinki, University of Helsinki and Helsinki University Hospital Comprehensive Cancer Center, Haartmaninkatu 8, P.O. Box 700, FIN-00290 Helsinki, Finland; email: satu.mustjoki@helsinki.fi.
References
Author notes
O.D. and S.M are joint last authors.
The processed single-cell RNA sequencing data is available at Zenodo (available at https://doi.org/10.5281/zenodo.13929103).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal