In this issue of Blood, Gibson et al1 have identified the impact of HLA alloimmunization in autologous gene-modified hematopoietic cell transplantation (GM-HCT) for transfusion-dependent thalassemia (TDT). Nickel et al have previously reported increased need for platelet and red cell transfusions after MSD HCT due to preexisting HLA and red cell antibodies in patients with sickle cell disease (SCD).2
Gibson et al report a similar observation in a cohort of 18 patients who underwent GM-HCT for TDT between 2015 and 2024; 13 underwent panel reflex antigen (PRA) testing, and 6 were PRAlow and 4 were PRAhigh. Patients with positive PRA were noted to have delayed platelet engraftment and higher transfusion needs. This has not been previously evaluated in GM-HCTs, as it is not a standard practice to check for HLA antibodies in patients undergoing autologous HCT. However, patients with hemoglobinopathies are at a significantly higher risk of allosensitization as they are usually exposed to multiple transfusions. This necessitates further investigation in larger studies to assess the utility of early identification of anti-HLA antibodies and its impact on outcomes.
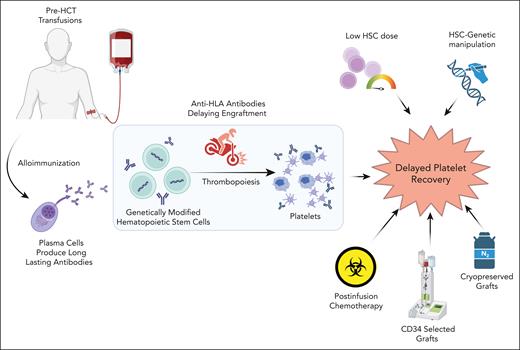
Anti-HLA antibodies (or donor-specific antibodies [DSAs]) have been known to adversely impact outcomes after both allo-HCT as well as solid organ transplants, often resulting in graft failure and other immunological complications.3,4 The underlying mechanism involves these antibodies interacting with donor HLA antigens and the resulting antigen-antibody complexes activating the complement pathway.5 Platelet engraftment often takes several weeks after an allo-HCT and is often further delayed in patients receiving post-HCT chemotherapy like methotrexate or cyclophosphamide.6 In patients who underwent GM-HCT for TDT with busulfan alone conditioning, platelet engraftment is often further delayed.7,8 This could be multifactorial; delayed megakaryopoiesis (and eventual platelet development) is noted after infusion of CD34-selected grafts (particularly with a low cell dose) and cryopreserved grafts.6 Though delayed platelet recovery has been previously reported to be associated with decreased survival after both autologous and allogeneic HCT,9 data are limited in the GM-HCT setting.
Screening for DSA is often performed prior to HLA-mismatched allo-HCTs. Recipients of a matched sibling donor (MSD) graft and GM-HCT traditionally are not screened for anti-HLA antibodies as the risk and potential adverse impact of these antibodies is anticipated to be negligible in these settings. However, in multiply transfused patients, such as those with hemoglobinopathies, there is frequent exposure to foreign antigens. Patients receiving GM-HCT for these disorders also receive multiple transfusions in preparation for stem cell collection and following graft infusion until successful red cell and platelet engraftment. Exposure to these antigens can often trigger an immune response with development of long-lasting antibodies and plasma cells.
There are several factors that could potentially confound platelet recovery in patients with TDT and SCD after graft infusion (see figure). These include complications such as infections, sinusoidal obstruction syndrome (SOS), and transplant-associated thrombotic microangiopathy. Patients with TDT are at a very high risk for developing SOS even with GM-HCT, which can result in increased platelet consumption. Delayed platelet recovery could significantly impact the outcomes in these patients. Pre-HCT detection of these antibodies provides an opportunity for immunomodulation prior to stem cell infusion. Preinfusion immunomodulation with interventions including immunoglobulins, rituximab, daratumumab, plasmapheresis, and splenectomy have shown the potential to offer additional benefit limiting the need of frequent transfusions after allo-HCT in individuals with DSA.10 This has the potential to improve the post-HCT course and further reduce the risk of bleeding complications. Once validated in larger studies, this can inform practice in early identification of these antibodies in GM-HCT patients and consider immunomodulation. This can hasten the platelet recovery and lessen the transfusion burden, thereby diminishing the risk of gene therapy-related complications. This becomes highly relevant as these GM-HCT therapies are now approved for SCD and TDT and are being used by a larger community of providers.
Risk factors for delayed platelet recovery after HCT. HSC, hematopoietic stem cell. Figure created with BioRender.com.
Risk factors for delayed platelet recovery after HCT. HSC, hematopoietic stem cell. Figure created with BioRender.com.
Conflict-of-interest disclosure: A.O.G. has received consultancy fees from Vertex Pharmaceuticals and has membership on the board of directors or advisory committees and research funding from Jazz Pharmaceuticals; is on the speakers bureaus for IEmerging Therapies Solutions; and Beam Therapeutics, Bluebird Bio, and Orchard therapeutics have provided research funding for ongoing clinical trial. A.S has received consultancy fees from Spotlight Therapeutics, Medexus Inc, Vertex Pharmaceuticals, Sangamo Therapeutics, Editas Medicine, and Pfizer; is a medical monitor for a Resource for Clinical Investigations in Blood and Marrow Transplantation supported Conditioning SCID-Infants Diagnosed Early clinical trial for which he receives financial compensation; has received research funding from CRISPR Therapeutics and honoraria from Vindico Medical Education and Blackwood Continuing Medical Education; and is the St. Jude Children’s Research Hospital site principal investigator for clinical trials of genome editing for sickle cell disease sponsored by Vertex Pharmaceuticals/CRISPR Therapeutics (NCT03745287), Novartis Pharmaceuticals (NCT04443907), and Beam Therapeutics (NCT05456880). The industry sponsors provide funding for the clinical trial, which includes salary support paid to the institution. A.S. has no direct financial interest in these therapies. These conflicts are managed through the Compliance Office at St. Jude Children’s Research Hospital, in accordance with their conflict-of-interest policy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal